Tepzz¥ 47 78A T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

August/September 1999 Volume 5, Number 4/5
T ECHNOLOGY, PRODUCTS, MA RKETS AND SERVICE OPPORTUNITIES A NEW MEDICINE PUBLICATION AUGUST/SEPTEMBER 1999 VOLUME 5, NUMBER 4/5 Flutamide 1090 STATE-OF-THE-ART IN THE MANAGEMENT OF CANCER Goserelin 1090 LUNG CANCER — PART II Leuprolide acetate 1091 SCREENING, DIAGNOSIS, AND Nilutamide 1091 CLASSIFICATION OTHER CHEMOTHERAPEUTICS 1091 LUNG CANCER SCREENING 1074 Alitretinoin 1091 Chest X-ray 1076 Anthracyclines 1092 Low-dose Helical Computed Tomography (CT) 1076 Daunorubicin 1092 Sputum Cytology 1078 Epirubicin 1092 Breath Analysis 1079 Liposomal daunorubicin 1092 Molecular Markers 1079 Liposomal doxorubicin 1092 Mitoxantrone 1094 PRESENTATION AND DIAGNOSIS 1080 Valrubicin 1094 Brush Cytology 1080 Busulfan 1094 Endoscopy/Bronchoscopy 1080 Carmustine Wafer 1094 Biopsy 1083 Etoposide Phosphate 1095 Noninvasive Imaging 1083 Flutarabine 1095 Computed tomography (CT) 1083 Gemcitabine 1095 Nuclear medicine 1083 Liposomal Cytarabine 1095 HISTOLOGY AND CLASSIFICATION 1084 Methoxsalen 1096 Porfimer Sodium 1096 SPECIAL REVIEW Temozolomide 1096 BIOLOGICALS 1098 ONCOLOGY TRENDS PRODUCT MARKETS — PART II Aldesleukin 1098 Denileukin Diftitox 1098 HORMONE MODULATING DRUGS 1084 Interferon α 1099 Antiestrogens 10854 Interferon α-2a 1099 Tamoxifene 1085 Interferon α-2b 1099 Raloxifene 1085 Rituximab 1099 Toremifene 1086 Trastuzumab 1101 Aromatase Inhibitors 1086 ADJUNCT THERAPIES 1101 Anastozole 1087 Amifostine 1101 Exemestane 1088 Colony Stimulating Factors 1102 Fadrozole 1088 Filgrastim 1102 Formestane 1089 Lenograstim 1102 Letrozole 1089 Sargramostim 1102 Antiandrogens 1089 Octreotide Acetate 1103 Bicalutamide 1090 Oprelvekin 1103 Cyproterone acetate 1090 Pamidronate Disodium 1104 COPYRIGHT © 1999 NEW MEDICINE INC. UNAUTHORIZED PHOTOCOPYING, DISTRIBUTION OR ELECTRONIC STORAGE IS PROHIBITED BY LAW. FUTURE ONCOLOGY AUGUST/SEPTEMBER 1999 VOLUME 5, NUMBER 4/5 ■ the Mayo Lung Project, initiated in 1971, that enrolled STATE-OF-THE-ART IN THE MANAGEMENT OF CANCER 9,211 males ≥45 years-of-age, who were heavy smok- ers. -

Targeting Fibrosis in the Duchenne Muscular Dystrophy Mice Model: an Uphill Battle
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.20.427485; this version posted January 21, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 Title: Targeting fibrosis in the Duchenne Muscular Dystrophy mice model: an uphill battle 2 Marine Theret1#, Marcela Low1#, Lucas Rempel1, Fang Fang Li1, Lin Wei Tung1, Osvaldo 3 Contreras3,4, Chih-Kai Chang1, Andrew Wu1, Hesham Soliman1,2, Fabio M.V. Rossi1 4 1School of Biomedical Engineering and the Biomedical Research Centre, Department of Medical 5 Genetics, 2222 Health Sciences Mall, Vancouver, BC, V6T 1Z3, Canada 6 2Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Minia 7 University, Minia, Egypt 8 3Developmental and Stem Cell Biology Division, Victor Chang Cardiac Research Institute, 9 Darlinghurst, NSW, 2010, Australia 10 4Departamento de Biología Celular y Molecular and Center for Aging and Regeneration (CARE- 11 ChileUC), Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, 8331150 12 Santiago, Chile 13 # Denotes Co-first authorship 14 15 Keywords: drug screening, fibro/adipogenic progenitors, fibrosis, repair, skeletal muscle. 16 Correspondence to: 17 Marine Theret 18 School of Biomedical Engineering and the Biomedical Research Centre 19 University of British Columbia 20 2222 Health Sciences Mall, Vancouver, British Columbia 21 Tel: +1(604) 822 0441 fax: +1(604) 822 7815 22 Email: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.01.20.427485; this version posted January 21, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. -

Preparation, Characterization, and in Vitro Enzymatic Hydrolysis in Biorelevant Media☆
European Journal of Pharmaceutical Sciences 92 (2016) 203–211 Contents lists available at ScienceDirect European Journal of Pharmaceutical Sciences journal homepage: www.elsevier.com/locate/ejps Modulating lipophilicity of rohitukine via prodrug approach: Preparation, characterization, and in vitro enzymatic hydrolysis in biorelevant media☆ Vikas Kumar a,b, Sonali S. Bharate a,⁎, Ram A. Vishwakarma b,c,⁎⁎ a Preformulation Laboratory, CSIR-Indian Institute of Integrative Medicine, Canal Road, Jammu 180001, India b Academy of Scientific and Innovative Research (AcSIR), CSIR-Indian Institute of Integrative Medicine, Canal Road, Jammu 180001, India c Medicinal Chemistry Division, CSIR-Indian Institute of Integrative Medicine, Canal Road, Jammu 180001, India article info abstract Article history: Rohitukine is a medicinally important natural product which has inspired the discovery of two anticancer clinical Received 26 May 2016 candidates. Rohitukine is highly hydrophilic in nature which hampers its oral bioavailability. Thus, herein our ob- Received in revised form 27 June 2016 jective was to improve the drug-like properties of rohitukine via prodrug-strategy. Various ester prodrugs were Accepted 11 July 2016 synthesized and studied for solubility, lipophilicity, chemical stability and enzymatic hydrolysis in plasma/ester- Available online 12 July 2016 ase. All prodrugs displayed lower aqueous solubility and improved lipophilicity compared with rohitukine, which was in accordance with the criteria of compounds in drug-discovery. The stability of synthesized prodrugs was Keywords: Prodrugs evaluated in buffers at different pH, SGF, SIF, rat plasma and in esterase enzyme. The rate of hydrolysis in all in- Drug discovery cubation media was dependent primarily on the acyl promoieties. Hexanoyl ester prodrug of rohitukine, 3d,was Rohitukine stable under chemical conditions; however it was completely hydrolyzed to rohitukine, in plasma and in esterase Solubility in 4 h. -

Stems for Nonproprietary Drug Names
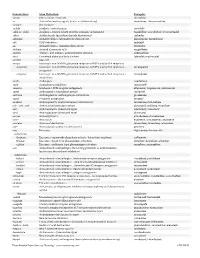
USAN STEM LIST STEM DEFINITION EXAMPLES -abine (see -arabine, -citabine) -ac anti-inflammatory agents (acetic acid derivatives) bromfenac dexpemedolac -acetam (see -racetam) -adol or analgesics (mixed opiate receptor agonists/ tazadolene -adol- antagonists) spiradolene levonantradol -adox antibacterials (quinoline dioxide derivatives) carbadox -afenone antiarrhythmics (propafenone derivatives) alprafenone diprafenonex -afil PDE5 inhibitors tadalafil -aj- antiarrhythmics (ajmaline derivatives) lorajmine -aldrate antacid aluminum salts magaldrate -algron alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol combined alpha and beta blockers labetalol medroxalol -amidis antimyloidotics tafamidis -amivir (see -vir) -ampa ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) subgroup: -ampanel antagonists becampanel -ampator modulators forampator -anib angiogenesis inhibitors pegaptanib cediranib 1 subgroup: -siranib siRNA bevasiranib -andr- androgens nandrolone -anserin serotonin 5-HT2 receptor antagonists altanserin tropanserin adatanserin -antel anthelmintics (undefined group) carbantel subgroup: -quantel 2-deoxoparaherquamide A derivatives derquantel -antrone antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine antineoplastics (arabinofuranosyl derivatives) fazarabine fludarabine aril-, -aril, -aril- antiviral (arildone derivatives) pleconaril arildone fosarilate -arit antirheumatics (lobenzarit type) lobenzarit clobuzarit -arol anticoagulants (dicumarol type) dicumarol -

TE INI (19 ) United States (12 ) Patent Application Publication ( 10) Pub
US 20200187851A1TE INI (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No .: US 2020/0187851 A1 Offenbacher et al. (43 ) Pub . Date : Jun . 18 , 2020 ( 54 ) PERIODONTAL DISEASE STRATIFICATION (52 ) U.S. CI. AND USES THEREOF CPC A61B 5/4552 (2013.01 ) ; G16H 20/10 ( 71) Applicant: The University of North Carolina at ( 2018.01) ; A61B 5/7275 ( 2013.01) ; A61B Chapel Hill , Chapel Hill , NC (US ) 5/7264 ( 2013.01 ) ( 72 ) Inventors: Steven Offenbacher, Chapel Hill , NC (US ) ; Thiago Morelli , Durham , NC ( 57 ) ABSTRACT (US ) ; Kevin Lee Moss, Graham , NC ( US ) ; James Douglas Beck , Chapel Described herein are methods of classifying periodontal Hill , NC (US ) patients and individual teeth . For example , disclosed is a method of diagnosing periodontal disease and / or risk of ( 21) Appl. No .: 16 /713,874 tooth loss in a subject that involves classifying teeth into one of 7 classes of periodontal disease. The method can include ( 22 ) Filed : Dec. 13 , 2019 the step of performing a dental examination on a patient and Related U.S. Application Data determining a periodontal profile class ( PPC ) . The method can further include the step of determining for each tooth a ( 60 ) Provisional application No.62 / 780,675 , filed on Dec. Tooth Profile Class ( TPC ) . The PPC and TPC can be used 17 , 2018 together to generate a composite risk score for an individual, which is referred to herein as the Index of Periodontal Risk Publication Classification ( IPR ) . In some embodiments , each stage of the disclosed (51 ) Int. Cl. PPC system is characterized by unique single nucleotide A61B 5/00 ( 2006.01 ) polymorphisms (SNPs ) associated with unique pathways , G16H 20/10 ( 2006.01 ) identifying unique druggable targets for each stage . -

(12) United States Patent (10) Patent No.: US 8.442,629 B2 Suzuki Et Al
USOO8442629B2 (12) United States Patent (10) Patent No.: US 8.442,629 B2 Suzuki et al. (45) Date of Patent: May 14, 2013 (54) IONTOPHORESIS PREPARATION FOR (56) References Cited TREATING BREAST CANCER AND/OR MASTITIS U.S. PATENT DOCUMENTS 7,384,418 B2 * 6/2008 Hung et al. ................ 604/890.1 (75) Inventors: Kenichi Suzuki, Fuji (JP); Makoto 2008: R ck 1939. aVleSs et al.. 600,547 Kanebako, Fuji (JP); Toshio Inagi, Fuji 2004/O152997 A1 ck 8, 2004 Davies . 600,547 (JP) 2004/02298.13 A1 11/2004 DiPiano et al. 2004/0253652 A1* 12/2004 Davies ......................... 435/723 (73) Assignee: Kowa Co., Ltd., Nagoya-shi (JP) 2004/0258747 A1 12/2004 Ponzoni et al. 2004/0267.189 A1* 12/2004 Mavor et al. .................... 604/20 c - r - 2005/020343.6 A1* 9, 2005 Davies .......................... 600,547 (*) Notice: Subject to any disclaimer, the term of this 2006/0177449 A1 8/2006 Matsumoto et al. patent is extended or adjusted under 35 2006/0184092 A1* 8, 2006 Atanasoska et al. ............ 604/20 U.S.C. 154(b) by 277 days. FOREIGN PATENT DOCUMENTS (21) Appl. No.: 12/988,047 EP 1 008365 A1 6, 2000 JP 2004-30014.7 10, 2004 JP 4000185 8, 2007 (22) PCT Filed: Apr. 17, 2009 WO 98.35722 8, 1998 (86). PCT No.: PCT/UP2009/001770 (Continued) S371 (c)(1), OTHER PUBLICATIONS (2), (4) Date: Oct. 15, 2010 U.S. Appl. No. 13/204.803, filed Aug. 8, 2011, Inagi, et al. (87) PCT Pub. No.: WO2009/128273 (Continued) PCT Pub. Date: Oct. -
Downloaded from Survive Nursing | Survivenursing.Com V20110426
Generic Stem Stem Definition Examples -abine (see -arabine, -citabine) decitabine -ac Anti-inflammatory agents (acetic acid derivatives) bromfenac; dexpemedolac -acetam See -racetam -actide Synthetic corticotropins seractide -adol or -aldol- Analgesics (mixed opiate receptor agonists/ antagonists) tazadolene; spiradolene; levonantradol -adox Antibacterials (quinoline dioxide derivatives) carbadox -afenone Antiarrhythmics (propafenone derivatives) alprafenone; diprafenone -afil PDE5 inhibitors tadalafil -aj- Antiarrhythmics (ajmaline derivatives) lorajmine -aldrate Antacid aluminum salts magaldrate -algron Alpha1 - and alpha2 - adrenoreceptor agonists dabuzalgron -alol Combined alpha and beta blockers labetalol; medroxalol -amivir (see -vir) -ampa Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) -ampanel Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) ; becampanel antagonists -ampator Ionotropic non-NMDA glutamate receptors (AMPA and/or KA receptors) ; forampator modulators -andr- Androgens nandrolone -anib Angiogenesis inhibitors semaxanib -anserin Serotonin 5-HT2 receptor antagonists altanserin; tropanserin; adatanserin -antel Anthelmintics (undefined group) carbantel -antrone Antineoplastics; anthraquinone derivatives pixantrone -apsel P-selectin antagonists torapsel -arabine Antineoplastics (arabinofuranosyl derivatives) fazarabine; fludarabine aril-, -aril, -aril- Antiviral (arildone derivatives) pleconaril; arildone; fosarilate -arit Antirheumatics (lobenzarit type) lobenzarit; clobuzarit -arol -

Datasheet Inhibitors / Agonists / Screening Libraries a DRUG SCREENING EXPERT
Datasheet Inhibitors / Agonists / Screening Libraries A DRUG SCREENING EXPERT Product Name : Prinaberel Catalog Number : TQ0149 CAS Number : 524684-52-4 Molecular Formula : C15H10FNO3 Molecular Weight : 271.24 Description: Prinaberel (ERB-041) is an effective and selective ERβ agonist and >200-fold selective for ERβ. Storage: 2 years -80°C in solvent; 3 years -20°C powder; Solubility DMSO ≥38mg/mL(140.1 mM) ( < 1 mg/ml refers to the product slightly soluble or insoluble ) In vitro Activity Treatment with ERβ selective estrogen agonists liquiritigenin and ERB-041 reduced the ability to invade a reconstituted basement membrane and to migrate in response to the cellular stimulus [1]. Pretreatment the PMs with ERB-041 resulted in significant inhibition of LPS-induced iNOS expression and NF-kappaB activation by preventing its nuclear translocation [3]. In vivo Activity Tumor numbers and volume were reduced by 60% and 84%, respectively, in the Erb-041-treated group as compared with UVB (alone) control. This inhibition in tumorigenesis was accompanied by a decrease in proliferating cell nuclear antigen (PCNA), cyclin D1, VEGF, and CD31, and an increase in apoptosis [2]. Reference 1. Hinsche O, et al. Estrogen receptor β selective agonists reduce invasiveness of triple-negative breast cancer cells. Int J Oncol. 2015 Feb;46(2):878-84. 2. Chaudhary SC, et al. Erb-041, an estrogen receptor-β agonist, inhibits skin photocarcinogenesis in SKH-1 hairless mice by downregulating the WNT signaling pathway. Cancer Prev Res (Phila). 2014 Feb;7(2):186-98. 3. Xiu-li W, et al. ERB-041, a selective ER beta agonist, inhibits iNOS production in LPS-activated peritoneal macrophages of endometriosis via suppression of NF-kappaB activation. -

Lisis: an Online Scientific Workflow System for Virtual Screening
Send Orders for Reprints to [email protected] Combinatorial Chemistry & High Throughput Screening, 2015, 18, 000-000 1 LiSIs: An Online Scientific Workflow System for Virtual Screening Christos C. Kannas*,1, Ioanna Kalvari2, George Lambrinidis3, Christiana M. Neophytou2, Christiana G. Savva2, Ioannis Kirmitzoglou2, Zinonas Antoniou1, Kleo G. Achilleos1, David Scherf 4, Chara A. Pitta2, Christos A. Nicolaou1, Emanuel Mikros3, Vasilis J. Promponas2, Clarissa Gerhauser4, Rajendra G. Mehta5, Andreas I. Constantinou2 and Constantinos S. Pattichis1 1Department of Computer Science, University of Cyprus, Nicosia, Cyprus 2Department of Biological Sciences, University of Cyprus, Nicosia Cyprus 3Department of Pharmaceutical Chemistry, Faculty of Pharmacy, National & Kapodistrian University of Athens, Athens, Greece 4Cancer Chemoprevention and Epigenomics Workgroup, German Cancer Research Center, Heidelberg, Germany Christos C. Kannas 5Illinois Institute of Technology (IIT) Research Institute, Chicago, Illinois, USA Abstract: Modern methods of drug discovery and development in recent years make a wide use of computational algorithms. These methods utilise Virtual Screening (VS), which is the computational counterpart of experimental screening. In this manner the in silico models and tools initial replace the wet lab methods saving time and resources. This paper presents the overall design and implementation of a web based scientific workflow system for virtual screening called, the Life Sciences Informatics (LiSIs) platform. The LiSIs platform consists of the following layers: the input layer covering the data file input; the pre-processing layer covering the descriptors calculation, and the docking preparation components; the processing layer covering the attribute filtering, compound similarity, substructure matching, docking prediction, predictive modelling and molecular clustering; post-processing layer covering the output reformatting and binary file merging components; output layer covering the storage component. -

Ep 2732820 A1
(19) TZZ ¥ Z_T (11) EP 2 732 820 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 21.05.2014 Bulletin 2014/21 A61K 31/675 (2006.01) A61K 31/66 (2006.01) A61K 31/47 (2006.01) (21) Application number: 14154215.9 (22) Date of filing: 14.04.2008 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Dalton, James T. HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT Lakeland, TN Tennessee 38002 (US) RO SE SI SK TR • Miller, Duane D. Collierville, TN Tennessee 38017-8799 (US) (30) Priority: 13.04.2007 US 785064 (74) Representative: Pearl Cohen Zedek Latzer Baratz (62) Document number(s) of the earlier application(s) in UK LLP accordance with Art. 76 EPC: 15 Old Bailey 08742872.8 / 2 136 815 London EC4M 7EF (GB) (71) Applicant: University of Tennessee Research Remarks: Foundation This application was filed on 06-02-2014 as a Knoxville, TN 37996-4122 (US) divisional application to the application mentioned under INID code 62. (54) Selective androgen receptor modulators for treating cancer (57) A composition comprising a compound of for- or its isomer, pharmaceutically acceptable salt, pharma- mula III characterized by the structure: ceutical product, hydrate, or N-oxide, and a chemother- apeutic agent. The chemotherapeutic agent may com- prise a taxane and a platinum compound, such as car- boplatin and docetaxel. The composition is for use in treating cancer in a subject. EP 2 732 820 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 732 820 A1 Description FIELD OF THE INVENTION 5 [0001] This invention provides SARM compounds and uses thereof in treating a variety of diseases or conditions in a subject, including, inter-alia, diabetes and associated diseases, such as, for example, chronic kidney disease, neph- ropathy, neuropathy and retinopathy; diabetes, insulin resistance, cachexia, cardiovascular disease and associated conditions such as, atherosclerosis, cerebrovascular disease, and others. -

Powerpoint Sunusu
PRODRUGS OF ALCOHOLS AND PHENOLS • Acylation or alkylation of alcohols or phenols could lead to a less polar prodrug while phosphorylation can lead to a more water soluble prodrug. A. Aliphatic and Aromatic Esters • Drugs containing hydroxyl groups, including alcohols and phenols can have a variety of physical/chemical properties that have advantages and disadvantages. • Esterification of the hydroxyl group has been one of the preferred prodrug strategies to mask polar groups within a drug molecule and thereby promote membrane permeability. • Acyl groups that have been incorporated to form promoieties for the hydroxyl group range from lower alkyl groups to long-chain fatty acids. • Valacyclovir is a water soluble L-valine ester prodrug of the HIV reverse transcriptase inhibitor acyclovir. • Valacyclovir was developed to increase the oral absorption and plasma levels of acyclovir. Increased plasma concentrations of acyclovir are important to maintain antiviral activity, especially in immunocompromissed patients and in the treatment of less sensitive viruses such as cytomegalovirus (CMV) and varicella zoster virus (VZV). • Valacyclovir is rapidly absorbed and converted to acyclovir by enzymatic hydrolysis in the intestine and liver. • It is hydrolysed by a valacyclovir hydrolase to produce acyclovir and L-valine. • A series of alkyl, cycloalkyl, and aryl ester prodrugs of the nonselective -adrenergic antagonist timolol have been prepared by esterifying the hydroxyl group of timolol. • All the prodrugs studied were more lipophilic than timolol and the most promising examples penetrated the cornea substantially better than timolol. • The rate of ester hydrolysis of alkyl, cycloalkyl, and aryl ester- prodrug was about equal in plasma and phosphate buffer, making it difficult to design a prodrug which is stable in vitro but will convert quickly to an active drug in vivo. -

(12) United States Patent (10) Patent No.: US 8,853,266 B2 Dalton Et Al
USOO8853266B2 (12) United States Patent (10) Patent No.: US 8,853,266 B2 Dalton et al. (45) Date of Patent: *Oct. 7, 2014 (54) SELECTIVE ANDROGEN RECEPTOR 3,875,229 A 4, 1975 Gold MODULATORS FOR TREATING DABETES 4,036.979 A 7, 1977 Asato 4,139,638 A 2f1979 Neri et al. 4,191,775 A 3, 1980 Glen (75) Inventors: James T. Dalton, Upper Arlington, OH 4,239,776 A 12/1980 Glen et al. (US): Duane D. Miller, Germantown, 4,282,218 A 8, 1981 Glen et al. TN (US) 4,386,080 A 5/1983 Crossley et al. 4411,890 A 10/1983 Momany et al. (73) Assignee: University of Tennessee Research 4,465,507 A 8/1984 Konno et al. F dati Kn ille, TN (US) 4,636,505 A 1/1987 Tucker Oundation, Knoxv1lle, 4,880,839 A 1 1/1989 Tucker 4,977,288 A 12/1990 Kassis et al. (*) Notice: Subject to any disclaimer, the term of this 5,162,504 A 11/1992 Horoszewicz patent is extended or adjusted under 35 5,179,080 A 1/1993 Rothkopfet al. U.S.C. 154(b) by 992 days. 5,441,868 A 8, 1995 Lin et al. 5,547.933 A 8, 1996 Lin et al. This patent is Subject to a terminal dis- 5,609,849 A 3/1997 Kung claimer. 5,612,359 A 3/1997 Murugesan et al. 5,618,698 A 4/1997 Lin et al. 5,621,080 A 4/1997 Lin et al. (21) Appl. No.: 11/785,064 5,656,651 A 8/1997 Sovak et al.