Monitoring Salt Marsh Vegetation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sea Level Driven Marsh Expansion in a Coupled Model of Marsh Erosion and Migration
W&M ScholarWorks VIMS Articles Virginia Institute of Marine Science 5-14-2016 Sea level driven marsh expansion in a coupled model of marsh erosion and migration Matthew L. Kirwan Virginia Institute of Marine Science DC Walters W. G. Reay Virginia Institute of Marine Science JA Carr Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Earth Sciences Commons Recommended Citation Kirwan, Matthew L.; Walters, DC; Reay, W. G.; and Carr, JA, "Sea level driven marsh expansion in a coupled model of marsh erosion and migration" (2016). VIMS Articles. 1418. https://scholarworks.wm.edu/vimsarticles/1418 This Article is brought to you for free and open access by the Virginia Institute of Marine Science at W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. PUBLICATIONS Geophysical Research Letters RESEARCH LETTER Sea level driven marsh expansion in a coupled 10.1002/2016GL068507 model of marsh erosion and migration Key Points: Matthew L. Kirwan1, David C. Walters1, William G. Reay1, and Joel A. Carr2 • Accelerated sea level rise leads to marsh expansion along gently 1Department of Physical Sciences, Virginia Institute of Marine Science, College of William and Mary, Gloucester Point, sloping, unhardened coasts 2 • Loss of marsh and natural flood Virginia, USA, Patuxent Wildlife Research Center, U.S. Geological Survey, Laurel, Maryland, USA protection is inevitable where barriers limit migration into uplands • Fluxes of organisms and sediment Abstract Coastal wetlands are among the most valuable ecosystems on Earth, where ecosystem services across adjacent ecosystems lead to such as flood protection depend nonlinearly on wetland size and are threatened by sea level rise and coastal increase in system resilience development. -

Early Holocene Wetland Succession at Lake Flixton (UK) and Its Implications for Mesolithic Settlement
Vegetation History and Archaeobotany https://doi.org/10.1007/s00334-019-00714-9 ORIGINAL ARTICLE Early Holocene wetland succession at Lake Flixton (UK) and its implications for Mesolithic settlement Barry Taylor1 Received: 29 May 2018 / Accepted: 23 January 2019 © The Author(s) 2019 Abstract This paper reports on new research into the timing and nature of post-glacial environmental change at Lake Flixton (North Yorkshire, UK). Previous investigations indicate a succession of wetland environments during the early Holocene, ultimately infilling the basin by ca 7,000 cal BP. The expansion of wetland environments, along with early Holocene woodland devel- opment, has been linked to changes in the human occupation of this landscape during the Mesolithic (ca 11,300–6,000 cal BP). However, our understanding of the timing and nature of environmental change within the palaeolake is poor, making it difficult to correlate to known patterns of Mesolithic activity. This paper provides a new record for both the chronology and character of environmental change within Lake Flixton, and discusses the implications for the Mesolithic occupation of the surrounding landscape. Keywords Mesolithic · Early Holocene · Plant macrofossils · Environmental change · Lake Flixton · Star Carr Introduction to the final Palaeolithic, terminal Palaeolithic and the early and late Mesolithic have also been recorded (Fig. 2) (Con- Since the late 1940s evidence for intensive phases of early neller and Schadla-Hall 2003). Early Mesolithic activity (ca prehistoric activity has been recorded around Lake Flix- 11,300–9,500 cal BP) was particularly intensive. Recent work ton in the eastern Vale of Pickering (North Yorkshire, UK) at Star Carr has shown that the site was occupied for ca (Fig. -

Field Nats News No. 301 Newsletter of the Field Naturalists Club of Victoria Inc
Field Nats News 301 Page 1 Field Nats News No. 301 Newsletter of the Field Naturalists Club of Victoria Inc. 1 Gardenia Street, Blackburn Vic 3130 Editor: Joan Broadberry 03 9846 1218 Telephone 03 9877 9860 Founding editor: Dr Noel Schleiger P.O. Box 13, Blackburn 3130 www.fncv.org.au Understanding Reg. No. A0033611X Our Natural World Newsletter email: [email protected] (Office email: [email protected]) Patron: The Honourable Linda Dessau, AC Governor of Victoria Office Hours: Monday and Tuesday 9.30 am - 4 pm. October 2019 NOTE EARLIER DEADLINE From the President It is nice to see some Spring sunshine although I have yet to see many invertebrates The deadline for FNN 302 will be moving about the garden. On the other hand there have been many small protoctists 10 am on Monday 30th September as to observe. Wendy Gare recently provided me with moss and water samples from the editor is hoping to go to Annuello. her garden pond in Blackburn and once again I have been delighted to see the ex- FNN will go to the printers on the 8th traordinary biodiversity of “infusoria” in a small urban pond. Microscopical exami- with collation on Tuesday 15th October nation revealed numerous amoeboids, ciliates, flagellates and a host of small arthro- pods and annelids including mites, copepods, fly larvae and tiny freshwater oligo- chaetes. Gastrotrichs, nematodes, rotifers, tardigrades and diatoms were also well Contents represented. Some of the protozoan organisms from Wendy’s pond are pictured be- low. There were also many spirochaete bacteria swimming about. -

Carr Slough Preservation and Tidal Wetland Restoration
Carr Slough Preservation and Tidal Wetland Restoration Carr Slough is a 103-acre privately owned tidal freshwater wetland connected to the Lower Columbia River near Prescott, Oregon in Columbia County (Figure 1). It is located at approximately River Mile 73 just upstream of Cottonwood Island and is situated between Highway 30 and the railway (Table 1). It has approximately 1.8 miles of channels, of which approximately one mile is comprised of straight channel drainage ditch with the remaining portions as an apparent natural channel with meanders and woody riparian vegetation. The site is mapped as entirely Rafton-Sauvie-Moag Complex soil type, a floodplain silty-clay alluvium derived of mixed sources and having extremely poor drainage. There is an opportunity to protect Carr Slough and its natural resource values for fish and wildlife habitat in perpetuity via the Natural Resources Conservation Service’s Wetland Reserve Program (WRP). The site has excellent value for a variety of species and important habitat types in the Lower Columbia, as detailed below, and would be an important site to preserve. There is also potential to undertake habitat improvement actions to increase the existing natural resource values of the site after the site has been preserved. Table 1. Site Location Information for Carr Slough Project Fourth Field Hydrologic Unit Code (HUC): Lower Columbia/Clatskanie 17080003 Project Fifth Field Hydrologic Unit Code (HUC): Beaver Creek/Columbia River 1708000302 Project T/R/S Location: T7N, R3W, Section 26 SE, 35 NE Project Latitude/Longitude: 46.0524381N, 122.8950261W USGS Quadrangle Map Name: Rainier County: Columbia Potential Resource Benefits & Preservation Value of Carr Slough There are multiple habitat types of value at Carr Slough, including freshwater tidal emergent wetland, shrub-scrub wetland, slough and freshwater tidal channel. -

CEDARBURG BOG WETLAND TYPES Kate Redmond Coniferous Bog, Coniferous Swamp, Fen, Lowland Hardwood Swamp, Marsh, Shrub Carr, Ephemeral Pond, Patterned Peatland
SOUTHEAST - 2 CEDARBURG BOG WETLAND TYPES Kate Redmond Coniferous bog, coniferous swamp, fen, lowland hardwood swamp, marsh, shrub carr, ephemeral pond, patterned peatland ECOLOGY & SIGNIFICANCE low strips of open sedge mat alternating with peat ridges of bog birch, leatherleaf, white cedar and tamarack. Plants Cedarburg Bog is the least disturbed large bog remaining common at the site include cranberry, bog birch, narrow- in southern Wisconsin. This wetland complex was once leaved sedge, bogbean, water horsetail, arrowgrass and part of a large glacial lake; today six lakes of varying size orchids as well as insectivorous plants like round-leaved and depth, all with high water quality, remain. The site’s • sundew, purple pitcher plant and bladderwort. More than OZAUKEE COUNTY 2500 acres support a number of different wetland plant 35 plant species at Cedarburg Bog are at or near the southern community types and an associated diversity of plants, extent of their range in Wisconsin. including many species that are regionally rare and are at the southern limits of their range here. This Wetland Gem Cedarburg Bog provides excellent habitat for both breeding also supports significant wildlife diversity including many and migrating birds. Nearly 300 species of birds have been amphibians, mammals and hundreds of birds. documented in the area, including 19 species that are near the southern extent of their range in Wisconsin. Breeding FLORA & FAUNA birds include Acadian Flycatcher, willow flycatcher, hooded This diverse wetland complex consists of extensive warbler, golden-crowned kinglet, Canada warbler, northern coniferous bog with a canopy of tamarack and black spruce waterthrush and white-throated sparrow. -

2020 Action Plan
Stream Stability and Water Quality 2018-2020 Schoharie Watershed Stream Management Program 2018 – 2020 Action Plan Photo of Batavia Kill Streambank Stabilization at Kastanis courtesy of Chris Langworthy (GCSWCD) NYCDEP Stream Management Program Greene County Soil & Water Conservation District 71 Smith Ave 907 County Office Building Kingston, NY 12401 Cairo, NY 12413 Dave Burns, ProjectGreene Manager County Soil & Water ConservationJeff Flack, Executive District Director 845.340.7850 907 County Office Building, Cairo518.622.6320 NY 12413 [email protected] [email protected] Stream Stability and Water Quality 2018-2020 Greene County Soil & Water Conservation District 907 County Office Building, Cairo NY 12413 Phone (518) 622-3620 Fax (518) 622-0344 To: David Burns, Project Manager, NYCDEP From: Jeff Flack, Executive Director, GCSWCD Date: May 15, 2018 Re: Schoharie Watershed Stream Management Program 2018-2020 Action Plan The Greene County Soil and Water Conservation District (GCSWCD) and the NYC Department of Environmental Protection (DEP) have collaborated with the Schoharie Watershed Advisory Committee (SWAC) to develop the 2018 – 2020 Action Plan. The Action Plan provides the Schoharie Watershed Stream Management Program’s activities, projects and programs that are planned for 2018-2020 as well as program accomplishments. The Action Plan is divided into key programmatic areas: A. Protecting and Enhancing Stream Stability and Water Quality B. Floodplain Management and Planning C. Highway and Infrastructure Management in Conjunction with Streams D. Assisting Streamside Landowners (Public and Private) E. Protecting and Enhancing Aquatic and Riparian Habitat F. Enhancing Public Access to Streams The Action Plan is updated and revised annually. This plan will be implemented from May 2018 – May 2020. -

CHEROKEE MARSH WETLAND TYPES Mario Quintano Marsh, Fen, Sedge Meadow, Low Prairie, Shrub Carr
SOUTHEAST - 3 CHEROKEE MARSH WETLAND TYPES Mario Quintano Marsh, fen, sedge meadow, low prairie, shrub carr ECOLOGY & SIGNIFICANCE areas of marsh habitat extend out into the river channel. Common herbaceous plants at the site include cattails, This urban Wetland Gem is the largest remaining wetland common bur-reed, river bulrush, softstem bulrush, in Dane County and a highly significant wetland within hardstem bulrush, sago pondweed, coontail, pickerel the Yahara River watershed. More than 2000 acres at weed and a variety of sedges and rushes. The site supports Cherokee Marsh, along both the east and west sides of DANE COUNTY some relic tamarack trees and several rare plant species, • the river, are protected by city, county and state agency including tufted bulrush, small yellow ladyslipper, white property owners. This extensive wetland complex is ladyslipper and glade mallow. characterized by steep side slopes and large flat expanses hosting marsh, fen, shrub carr, sedge meadow, and one of Many mammals, amphibians, reptiles and birds use the largest low prairies in the region. Cherokee Marsh not Cherokee Marsh. A wide diversity of birds nest in or only serves as excellent wildlife habitat, but also provides migrate through the area, including northern harrier, services like water quality protection and flood attenuation short-eared owl, great horned owl, American egret, great to the City of Madison downstream. The City has intensive blue heron, green heron, sandhill crane, marsh wren, and ongoing marsh restoration efforts that are helping to sedge wren, swamp sparrow, belted kingfisher, and bring back hundreds of lost acres. The site also provides many species of ducks. -

Botanical and Ecological Inventory of Peatland Sites on the Medicine Bow National Forest
Botanical and Ecological Inventory of Peatland Sites on the Medicine Bow National Forest Prepared for Medicine Bow National Forest By Bonnie Heidel and Scott Laursen Wyoming Natural Diversity Database University of Wyoming P.O. Box 3381 Laramie, WY 82071 FS Agreement No. 02-CS-11021400-012 June 2003 ABSTRACT Peatlands are specialized wetland habitats that harbor high concentrations of Wyoming plant species of special concern. Intensive botanical and ecological inventories were conducted at five select peatland sites on Medicine Bow National Forest to further document the vascular flora, update information on plant species of special concern, initiate documentation of the bryophyte flora composition, and to document the vegetation associations. This provides a preliminary summary of peatland botanical and ecological resources on Medicine Bow National Forest, data for comparison between sites, and both floristic and vegetation plot datasets for comparison between Medicine Bow National Forest and Shoshone National Forest where parallel studies were undertaken. It might be used for more more extensive systematic inventories of peatlands and their associated botanical and ecological attributes across the Forest, or related efforts to evaluate watershed, wildlife, and other values associated with peatlands. ACKNOWLEDGEMENTS John Proctor (USFS Medicine Bow NF) contributed to fieldwork and provided Forest Service coordination that made this project a reality. Judy Harpel (USFS) made the moss species identifications for a segment of moss specimens, and provided encouragement in bryophyte research. George Jones (WYNDD) and Kathy Roche (USFS Medicine Bow NF) provided literature and comments on site selection and study aspects. Gary Beauvais provided administrative support. Chris Hiemstra and Mark Lyford (UW) provided study site data. -

Wms92 Southern Basin Wet Meadow/Carr Factsheet
WET MEADOW/CARR SYSTEM WMs92 Southern Floristic Region Southern Basin Wet Meadow/Carr Open wetlands dominated by dense cover of broad-leaved sedges. Typically present in small, closed, shallow basins isolated from groundwater inputs. Vegetation Structure & Composition Description is based on summary of vegetation data from 28 plots (relevés). • Mosses are typically absent or sparse, although the weedy and shade-tolerant spe- cies Drepanocladus aduncus is commonly present beneath dense sedge tussocks • Graminoid cover is continuous (75–100%); typically dominated by slough sedge (Carex atherodes), sometimes with whitetop (Scolo- chloa festucacea), bluejoint (Calamagrostis canadensis), prairie cordgrass (Spartina pectinata), red-stalked spikerush (Eleocharis palustris), or lake sedge (Carex lacustris). • Forb cover is variable, ranging from absent to sparse (< 25%); common species include water smartweed (Polygonum amphibium), cattails (Typha spp.), common mint (Men- tha arvensis), rough bugleweed (Lycopus asper), water parsnip (Sium suave), and swamp milkweed (Asclepias incarnata). • Shrub cover is sparse; pussy willow (Salix discolor), slender willow (S. petiolaris), and red-osier dogwood (Cornus sericea) may be present. • Note: WMs92 has become uncommon across much of its range as a result of invasion by non-native species, especially reed canary grass (Phalaris arundinacea), following alterations in wetland hydrology. Landscape Setting & Soils WMs92 occurs primarily in shallow wetland basins and rarely along shores of lakes and rivers. Surface water is derived from rainfall and surface flow; in general, sites are isolated from lateral groundwater flow. Water chemistry is basic (pH > 7) because of influence of calcareous soils. Relatively low precipitation in the prairie region and high rates of evaporation result in higher salinity in this community compared with other Wet Meadow/Carr communities in Minnesota. -
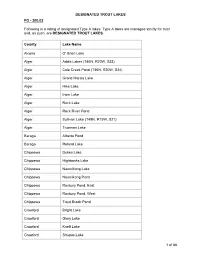
Designated Trout Lakes and Streams
DESIGNATED TROUT LAKES FO - 200.02 Following is a listing of designated Type A lakes. Type A lakes are managed strictly for trout and, as such, are DESIGNATED TROUT LAKES. County Lake Name Alcona O' Brien Lake Alger Addis Lakes (T46N, R20W, S33) Alger Cole Creek Pond (T46N, R20W, S24) Alger Grand Marais Lake Alger Hike Lake Alger Irwin Lake Alger Rock Lake Alger Rock River Pond Alger Sullivan Lake (T49N, R15W, S21) Alger Trueman Lake Baraga Alberta Pond Baraga Roland Lake Chippewa Dukes Lake Chippewa Highbanks Lake Chippewa Naomikong Lake Chippewa Naomikong Pond Chippewa Roxbury Pond, East Chippewa Roxbury Pond, West Chippewa Trout Brook Pond Crawford Bright Lake Crawford Glory Lake Crawford Kneff Lake Crawford Shupac Lake 1 of 86 DESIGNATED TROUT LAKES County Lake Name Delta Bear Lake Delta Carr Lake (T43N, R18W, S36) Delta Carr Ponds (T43N, R18W, S26) Delta Kilpecker Pond (T43N, R18W, S11) Delta Norway Lake Delta Section 1 Pond Delta Square Lake Delta Wintergreen Lake (T43N, R18W, S36) Delta Zigmaul Pond Gogebic Castle Lake Gogebic Cornelia Lake Gogebic Mishike Lake Gogebic Plymouth Lake Houghton Penegor Lake Iron Deadman’s Lk (T41N, R32W, S5 & 8) Iron Fortune Pond (T43N, R33W, S25) Iron Hannah-Webb Lake Iron Killdeer Lake Iron Madelyn Lake Iron Skyline Lake Iron Spree Lake Isabella Blanchard Pond Keweenaw Manganese Lake Keweenaw No Name Pond (T57N, R31W, S8) Luce Bennett Springs Lake Luce Brockies Pond (T46N, R11W, S1) 2 of 86 DESIGNATED TROUT LAKES County Lake Name Luce Buckies Pond (T46N, R11W, S1) Luce Dairy Lake Luce Dillingham -

No Slide Title
Considering scale when assessing wetland methane emissions: Wetland forest soils versus wetland forests. Vincent Gauci & Sunitha Pangala CEPSAR, Department of Environment, Earth and Ecosystems, The Open University, UK 9th INTECOL International Wetlands Conference Syposium: Measurement of Greenhouse Gas Emissions from Wetlands Orlando (Florida), 5th June 2012 Broadleaf evergreen forest • Greenhouse study on pot grown tree seedlings in rice soil. • Emission pathways not identified (assumed to be transpiration in leaves). • Emissions scaled to the globe using LAI • Estimate that wetland trees contribute around 60Tg CH4 Gauci et al 2010 (Atmos. Env.) 1.961 1.959 1.957 1.955 1.953 1.951 [CH4]ppm 1.949 1.947 1.945 1.943 0 200 400 600 800 1000 1200 1400 1600 time seconds Methane emissions from alder tree stems Methods: Approach to understanding tree contributions to forested wetland CH4 emissions at the ecosystem scale. • A mesocosm study to examine the controls and pathways of tree emission. • A one-year in situ study in a temperate alder carr ecosystem • An in situ study in tropical peat swamp forest in Kalimantan Mesocosm study: Alder saplings Mesocosms: What is the methane exit pathway? 2.9 ) y = 0.560x + 0.786 1 - R² = 0.774, P < 0.001 hr 2.6 2 - 2.3 flux (mg m 4 2.0 1.7 stem CH stem 1.4 0.8 1.3 1.8 2.3 2.8 stem lenticel density (lenticels/cm2) Significant relationship also observed between CH4 emissions and porewater [CH4]. No relationship observed between emissions and leaf area Pangala et al. (New Phytologist under revision). -

Eastern Broadleaf Forest Province, Wet Meadow/Carr System Summary
WM Wet Meadow/Carr System photo by E.R. Rowe MN DNR Rowe E.R. by photo Becker County, MN General Description Wet Meadow/Carr (WM) communities are graminoid- or shrub-dominated wetlands that are subjected annually to moderate inundation following spring thaw and heavy rains and to periodic drawdowns during the summer. The dominant graminoids are broad- leaved species such as lake sedge (Carex lacustris), tussock sedge (C. stricta), and bluejoint (Calamagrostis canadensis). Shrubs such as willows (Salix spp.) and dog- woods (Cornus spp.) are likely to be dominant on drier sites. Peak water levels are high and persistent enough to prevent trees (and often shrubs) from becoming established. However, there may be little or no standing water present during much of the growing season. As a result, the substrate surface alternates between aerobic and anaerobic conditions. Any organic matter that accumulates over time is usually oxidized during pe- riodic drawdowns and may even burn during severe droughts. Soils range from mineral soils to muck and peat. Silt from flooding sometimes is intermixed with organic matter in muck or peat soils. Although WM communities can be present on deep peat, they are not “peat-accumulating” communities. Rather, the peat was usually formed previously on the site by a peat-producing community, such as a Forested Rich Peatland, that was flooded by beaver activity and converted to a WM community. Deep peat may also be present in some WM communities because of debris that has been transported into the wetland, forming sedimentary peat. Because surface water is derived from runoff, stream flow, or groundwater, it is circumneutral (pH 6.0–8.0) and has high mineral and nutrient content.