Butvar Properties and Uses Brochure
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Butvar Properties and Uses
Pub. No. 2008084E (Supersedes 2008084D) Butvar ® POLYVINYL BUTYRAL RESIN PROPERTIES & USES Coatings Performance Materials by CONTENTS Introduction 1 Properties 2 Chemistry 2 Properties Tables 3 Product Types 6 Butvar:The Right Resin Solutions 6 Compatibility 13 Insolubizing Reactions 15 Reaction With Phenolics 15 Reaction With Epoxies 15 Reaction With Dialdhehydes 16 Reaction With Isocyanates 16 Reaction With Melamines 16 Applications 17 Wire Enamels 17 Surface Coatings 17 Wash Primers 17 Military Specification Wash Primers 17 Non-specification Wash Primers: B-1030 With Butvar 18 Single Package Wash Primer: B-1011 With Butvar 18 Chromate-free Wash Primers With Butvar 19 Metal Coatings 20 Wood Finishes 21 Protective Wash Coats and Sealers 21 Knot Sealers 21 Adhesives 22 Structural Adhesives 22 Phenolic Resins 22 Epoxies and Other Thermosetting Resins 22 High-strength Bonding Procedure 23 Performance Characteristics 23 Adhesive Strengths 23 Hot Melt Adhesives 24 Textile Coatings 24 Advantages as Textile Coating 24 Ceramic Binder Applications 25 Tape Casting 26 Thick Films 26 Toners and Printing Inks 27 Storage and Handling 28 Storage 28 Toxicity and FDA Status 28 Quality Control 28 Material Sources Inside Back Cover Worldwide Sales Offices Back Cover Enfocus Software - Customer Support INTRODUCTION olyvinyl butyral resins are employed in a wide USES array of industrial and commercial applications. Some of the applications in which Butvar is a vital PThese unique resins offer impressive performance, ingredient include: as well as outstanding versatility. Ceramic binders Butvar® polyvinyl butyral resins have a combination of Inks/dry toners properties that make them a key ingredient in a variety Wood coatings of successful formulations. -

2 Types of Noncrystalline Polymers
2 Types of Noncrystalline Polymers 1. Glassy polymer highly interpenetrated/entangled 2. Rubbery polymers random Gaussian coils Glass Transition Temperature εij Two viewpoints: • Increasing T: When kT > magnitude of εij, the thermal fluctuations can overcome local intermolecular bonds and the frozen (“glassy”) structure becomes “fluid-like”. • Decreasing T: As the temperature is lowered and T approaches Tg, the viscosity increases to ∞ and the material becomes “solid” Free Volume Theory of Tg Free volume, VF – extra space beyond what is present in an ordered crystalline packing (beyond the interstitial volume). VF(T) ≡ V(T) – V0(T) • V0 is occupied specific volume of atoms or molecules in the xline state and the spaces between them: ~ VXL. V(T) α • VF increases as T increases due to the Rate of cooling l difference in the thermal expansion V (T) αg XL coefficients (αg vs αl). FAST SLOW • V0(T) ≈ VXL(T) ↔ can take αg ≈αXL dV •V(T) = V (T ) + (T-T ) F T > T F F g g dT g Tg T • define fractional free volume, fF:Vf/V fF(T) = fF(Tg) + (T-Tg)αf αf = αl – αg Viewpoint: Tg occurs when available free volume drops below critical threshold for structural rearrangement [VITRIFICATION POINT], structure “jams up”. Tg Values of Amorphous Materials Table of representative amorphous solids, their bonding types, and their glass transition temperatures removed due to copyright restrictions. See Table 2.2 in Allen, S. M., and E.L. Thomas. The Structure of Materials. New York, NY: J. Wiley & Sons, 1999. Tg for Selected Polymers Glass Transition Temperature -

Monsanto Company in Microsoft Word Format Together with a Copy of the Transmittal Letter That Accompanies the Filing of Two Paper Copies of the Submission
From: Letzler, Kenneth [mailto:[email protected]] Sent: Thursday, December 31, 2009 1:03 PM To: ATR-Agricultural Workshops Subject: Comment Attached please find a comment submitted on behalf of Monsanto Company in Microsoft Word format together with a copy of the transmittal letter that accompanies the filing of two paper copies of the submission. _____________________________ U.S. Treasury Circular 230 Notice Any U.S. federal tax advice included in this communication (including any attachments) was not intended or written to be used, and cannot be used, for the purpose of (i) avoiding U.S. federal tax-related penalties or (ii) promoting, marketing or recommending to another party any tax-related matter addressed herein. _____________________________ This communication may contain information that is legally privileged, confidential or exempt from disclosure. If you are not the intended recipient, please note that any dissemination, distribution, or copying of this communication is strictly prohibited. Anyone who receives this message in error should notify the sender immediately by telephone or by return e-mail and delete it from his or her computer. ---------------------------------------------------------------------- For more information about Arnold & Porter LLP, click here: http://www.arnoldporter.com Competition and Innovation in American Agriculture A Response to the American Antitrust Institute’s “Transgenic Seed Platforms: Competition Between a Rock and a Hard Place?” Submitted on Behalf of Monsanto Company In Response to the Request for Comments by the United States Department of Agriculture and United States Department of Justice, Antitrust Division, in Connection with Their Hearings on “Agriculture and Antitrust Enforcement Issues in Our 21st Century Economy” Vandy Howell, Ph.D. -

Green Tide Monsanto
Green Tide http://www.zmag.org/zmag/articles/mar99tokar.htm Monsanto: A Checkered History Who should choose our technologies? By Brian Tokar Headquartered just outside St. Louis, Missouri, the Monsanto Chemical Company was founded in 1901 by John Francis Queeny. Queeny, a self- educated chemist, brought the technology to manufacture saccharin, the first artificial sweetener, from Germany to the United States. In the 1920s, Monsanto became a leading manufacturer of sulfuric acid and other basic industrial chemicals, and is one of only four companies to be listed among the top ten U.S. chemical companies in every decade since the 1940s. By the 1940s, plastics and synthetic fabrics had become a centerpiece of Monsanto’s business. In 1947, a French freighter carrying ammonium nitrate fertilizer blew up at a dock 270 feet from Monsanto’s plastics plant outside Galveston, Texas. More than 500 people died in what came to be seen as one of the chemical industry’s first major disasters. The plant was manufacturing styrene and polystyrene plastics, which are still important constituents of food packaging and various consumer products. In the 1980s the U.S. Environmental Protection Agency (EPA) listed polystyrene as fifth in its ranking of the chemicals whose production generates the most total hazardous waste. In 1929, the Swann Chemical Company, soon to be purchased by Monsanto, developed polychlori- nated biphenyls (PCBs), which were widely praised for their nonflammability and extreme chemical stability. The most widespread uses were in the electrical equipment industry, which adopted PCBs as a nonflammable coolant for a new generation of transformers. -

A Global Citizens Report on the State of Gmos Synthesis Report !"#$%&'$#&(#)')$ "*+$,'$-.'!"#+
!"#$%&'$#&(#)')$ "*+$,'$-.'!"#+ Published by A Global Citizens Report on the State of GMOs Synthesis Report !"#$%&'$#&(#)')$ "*+$,'$-.'!"#+ !"#$%&'$"()*)+,-."/,0%1*" %-"*2,"3*'*,"%4"#56."7 8'$.,"91%:).,.;"8')$,<"=,>2-%$%?),. 3@-*2,.)."/,0%1* Coordinated by Vandana Shiva, Navdanya Debbie Barker, Centre for Food Safety Caroline Lockhart, Navdanya International Front page cartoon: Sergio Staino Layout, production and printing: SICREA srl, Florence Contact: [email protected] Available for download at www.navdanyainternational.it www.navdanya.org Thanks go to all those who readily contributed in the realization of this report, particularly Sergio Staino, creator of the cover cartoon, Maurizio Izzo of Sicrea for production and Massimo Orlandi for press and communications. Thanks also go to Christina Stafford and interns Tara McNerney and Tanvi Gadhia of Center for Food Safety (CFS) and Sulakshana Fontana, Elena Bellini and Filippo Cimo of Navdanya International for their diligent coordination, editing and translation efforts. Final thanks also go to Giannozzo Pucci, Maria Grazia Mammuccini and Natale Bazzanti for their cooperation and assistance in realizing this report. This publication is printed on ecological paper SYMBOL FREELIFE SATIN ECF *$%/012/$-3435678$)690:4$ 07$4;6$+4246$0<$%&'8$=$ >2/86$(:0?3868@$>23/6A$!6B;70/0C368 Coordinated by Navdanya and Navdanya International, the International Commission on the Future of Food and Agriculture, with the participation of The Center for Food Safety (CFS) Contributing organizations: -

Who Will Control the Green Economy? ETC Group, 2011
!"#$%&''$(#)*+#'$*",$ -+,,)$.(#)#/01 23$4#5,+)/,)*3$6+,67+,$*#$37)(*&#)$7$-+,,) .(#)#/0 7*$8	:;<$.=>$-+#?6$6+#5&@,3$ 7)$?6@7*,$#)$(#+6#+7*,$6#%,+$7)@$ %7+)3$*"7*$*",$A?,3*$*#$(#)*+#'$B&#/733$ %&''$6,+6,*?7*,$*",$-+,,@ .(#)#/0C %%%C,*(4+#?6C#+4 “We are told by men of science that all the venture of mariners on the sea, all that counter-marching tribes and races that confounds old history with its dust and rumour, sprang from nothing more abstruse than the laws of supply and demand, and a certain natural instinct for cheap rations. To any one thinking deeply, this will seem a dull and pitiful explanation.” —Robert Louis Stevenson, Will o’ the Mill, 1901 “As long as the maximization of profit remains the cornerstone of acquisitive society and capitalist economy, corporations will retain their interest in scarcity as a creator of economic value.” —German-born economist, Erich W. Zimmermann, in World resources and industries: a functional appraisal of the availability of agricultural and industrial materials, 1933 2(D)#%',@4,/,)*3 All original artwork, including the cover illustration, “BioMassters: The Board Game,” and report design by Shtig. ETC Group gratefully acknowledges the financial support of SwedBio (Sweden), HKH Foundation (USA), CS Fund “Trickle Down” by Adam Zyglis used with permission. (USA), Christensen Fund (USA), Heinrich Böll Who Will Control the Green Economy? is ETC Group Foundation (Germany), the Lillian Goldman Charitable Communiqué no. 107. Trust (USA), Oxfam Novib (Netherlands) and the Norwegian Forum for Environment and Development. November 2011 ETC Group is solely responsible for the views expressed in All ETC Group publications are available free of charge this document. -
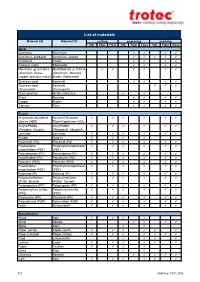
List of Materials
List of materials Material EN Material DE cutting engraving marking CO2 Fiber Flexx CO2 Fiber Flexx CO2 Fiber Flexx Metal Aluminum Aluminium Aluminum, anodized Aluminium, eloxiert Chromium Chrom Precious metal Edelmetall Metal foils up to 0.5mm Metallfolie bis zu 0,5mm (Aluminum, Brass, (Aluminium, Messing, Copper, precious metal) Kupfer, Edelmetall) Stainless steel Edelstahl Stainless steel Edelstahl (Thermark®) (Thermark®) Metal, painted Metall, lackiertes Brass Messing Copper Kupfer Titanium Titan Plastic Acrylonitrile butadiene Acrylnitril-Butadien- styrene (ABS) Styrol-Copolymer (ABS) Acrylic/PMMA Acryl/PMMA (Plexiglas, Altuglas, (Plexiglas®, Altuglas®, LaminatePerspex) LaminatePerspex®) Rubber Gummi Polyamide (PA) Polyamid (PA) Polybutylene Polybutylenterephthalat terephthalate (PBT) (PBT) Polycarbonate (PC) Polycarbonat (PC) Polyethylene (PE) Polyethylen (PE) Polyester (PES) Polyester (PES) Polyethylene Polyethylenterephthalat terephthalate (PET) (PET) Polyimide (PI) Polyimid (PI) Polyoxymethylene Polyoxymethylen (POM) -Delrin® (POM) - Delrin® Polypropylene (PP) Polypropylen (PP) Polyphenylene sulfide Polyphenylensulfid (PPS) (PPS) Polystyrene (PS) Polystyrol (PS) Polyurethane (PUR) Polyurethan (PUR) Foam Schaumstoff Miscellanious Wood Holz Mirror Spiegel Stone Stein Paper (white) Papier (weiß) Paper (colored) Papier (färbig) Food Lebensmittel Leather Leder -

Seattle V. Monsanto Complaint
Case 2:16-cv-00107-RSL Document 31 Filed 05/04/16 Page 1 of 27 1 THE HONORABLE ROBERT S. LASNIK 2 3 4 5 6 7 UNITED STATES DISTRICT COURT 8 WESTERN DISTRICT OF WASHINGTON 9 CITY OF SEATTLE, a municipal corporation ) CASE NO. 2:16-cv-00107-RSL located in the County of King, State of ) 10 Washington, ) PLAINTIFF’S FIRST AMENDED ) COMPLAINT 11 Plaintiffs, ) ) 12 v. ) ) 13 MONSANTO COMPANY, ) SOLUTIA INC., and ) 14 PHARMACIA CORPORATION, and DOES 1 ) through 100, ) 15 ) Defendants. ) 16 ________________________________________ I. INTRODUCTION 17 1. Polychlorinated biphenyls (or “PCBs”) are man-made chemical compounds that 18 have become notorious as global environmental contaminants — found in bays, oceans, rivers, 19 streams, soil, and air. As a result, PCBs have been detected in the tissues of all living beings on 20 earth including all forms of marine life, various animals and birds, plants and trees, and humans. 21 2. The extent of environmental PCB contamination is troubling because PCBs cause 22 a variety of adverse health effects. In humans, PCB exposure is associated with cancer as well as 23 serious non-cancer health effects, including effects on the immune system, reproductive system, PLAINTIFF’S FIRST AMENDED COMPLAINT - 1 Peter S. Holmes Seattle City Attorney 701 5th Avenue, Suite 2050 Seattle, WA 98104-7097 (206) 684-8200 Case 2:16-cv-00107-RSL Document 31 Filed 05/04/16 Page 2 of 27 1 nervous system, endocrine system and other health effects. In addition, PCBs destroy 2 populations of fish, birds, and other animal life. 3 3. -

World Resources Institute the Monsanto Company
World Resources Institute Sustainable Enterprise Program A program of the World Resources Institute The Monsanto Company: Quest for Sustainability (A) “Biotechnology represents a potentially sustainable For more than a decade, WRI's solution to the issue, not only of feeding people, but of providing Sustainable Enterprise Program (SEP) the economic growth that people are going to need to escape has harnessed the power of business to poverty…… [Biotechnology] poses the possibility of create profitable solutions to leapfrogging the industrial revolution and moving to a post- environment and development industrial society that is not only economically attractive, but challenges. BELL, a project of SEP, is also environmentally sustainable.i ” focused on working with managers and academics to make companies --Robert Shapiro, CEO, Monsanto Company more competitive by approaching social and environmental challenges as unmet market needs that provide Upon his promotion to CEO of chemical giant The business growth opportunities through Monsanto Company in 1995, Robert Shapiro became a vocal entrepreneurship, innovation, and champion of sustainable development and sought to redefine the organizational change. firm’s business strategy along principles of sustainability. Shapiro’s rhetoric was compelling. He captured analysts’ Permission to reprint this case is attention with the specter of mass hunger and environmental available at the BELL case store. degradation precipitated by rapid population growth and the Additional information on the Case -

Use of a Crosslinking Agent, Crosslinked Polymer, and Uses Thereof
(19) TZZ Z_T (11) EP 2 452 970 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C08K 5/09 (2006.01) B32B 27/30 (2006.01) 21.01.2015 Bulletin 2015/04 C08L 29/04 (2006.01) C08L 101/00 (2006.01) G02B 5/30 (2006.01) C08K 5/098 (2006.01) (2006.01) (21) Application number: 12154871.3 C08K 5/10 (22) Date of filing: 29.08.2008 (54) Use of a crosslinking agent, crosslinked polymer, and uses thereof Verwendung eines Vernetzungsmittels, vernetztes Polymer und Verwendungen davon Utilisation d’un agent de réticulation, polymère réticulé et utilisations associées (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Kageyama, Hideki HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT Osaka-shi, Osaka 530-0018 (JP) RO SE SI SK TR • Tanaka, Shinichi Osaka-shi, Osaka 530-0018 (JP) (30) Priority: 31.08.2007 JP 2007225012 • Ono, Hiroyuki 18.03.2008 JP 2008069645 Osaka-shi, Osaka 530-0018 (JP) 04.04.2008 JP 2008098282 • Kuruma, Akiko 11.04.2008 JP 2008103494 Osaka-shi, Osaka 530-0018 (JP) 24.04.2008 JP 2008114149 24.04.2008 JP 2008114150 (74) Representative: Kuhnen & Wacker Patent- und Rechtsanwaltsbüro (43) Date of publication of application: Prinz-Ludwig-Straße 40A 16.05.2012 Bulletin 2012/20 85354 Freising (DE) (62) Document number(s) of the earlier application(s) in (56) References cited: accordance with Art. 76 EPC: WO-A1-2005/087711 JP-B2- 3 612 124 08828502.8 / 2 189 496 US-A1- 2004 102 586 (73) Proprietor: The Nippon Synthetic Chemical • DATABASE WPI Week 198020 Thomson Industry Co., Ltd. -

BASEL CONVENTION PLASTICS AMENDMENTS Institute of Scrap Recycling Industries (ISRI)
BASEL CONVENTION PLASTICS AMENDMENTS Institute of Scrap Recycling Industries (ISRI) Adina Renee Adler Assistant Vice President, International Affairs ©2019 Institute of Scrap Recycling Industries, Inc. All Rights Reserved The Driver 2 Basel Convention Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal Adopted 22 March 1989 Entered into force on 5 May 1992 Currently has 187 parties The United States is not a party. OCTOBER 2019 | ISRI.ORG 3 ©2019 ISRI, Inc. Basel Convention Basel Convention on the Control of Transboundary Movements of Hazardous Wastes and Their Disposal Subjects hazardous wastes or other wastes with hazardous constituents / exhibiting hazardous characteristics to a “prior informed consent” (PIC) procedure before the material can be shipped across national borders. The PIC gives the receiving country the right of refusal if proper recycling or disposal operations do not exist. OCTOBER 2019 | ISRI.ORG 4 ©2019 ISRI, Inc. Basel Convention 14th Conference of the Parties (COP) held every two years to direct work on implementing the convention and addressing emerging issues. OCTOBER 2019 | ISRI.ORG 5 ©2019 ISRI, Inc. Norway’s Proposal Amend the following annexes so that trade controls are 1 put into place for certain plastics: Annex II: Non-hazardous, hard-to-recycle plastics Annex VIII: Hazardous plastics Annex IX: Non-hazardous, easy-to-recycle plastics (exempt from BC) Create a Partnership for Plastic Waste that would involve public and private stakeholders to find possible solutions 2 to plastic leakage. Terms of Reference negotiated and adopted. It will lead to non- binding initiatives to support developing country improvements to minimization, collection and proper handling of end-of-life plastics. -

Polyvinylbutyral HVB-0001
Technical Information Polyvinylbutyral HVB-0001 Description: HVB-0001 is high viscosity thermoplastic Polyvinylbutyral Resin combining a very large surface area with medium molecular weight. Its sensorial properties are outstanding. The product is soluble in solvents like Alcohols or Glycol Ethers, it is compatible with Esters and Ketones. HVB-0001 shows excellent compatibility with other resins like Nitrocellulose, Epoxy Resins, Epoxy Alkyds, Phenolic Resins, Polyurethanes, Melamine-Formaldehyde Resins, Ketonic Resins and Polyamides. With its free hydroxyl groups the material is chemically reactive. Application: Printing Inks and lacquers: HVB-0001 is an ideal choice for formation of printing inks for flexible packaging (Flexographic & Gravure application). The product offers good water resistance combined with good blocking resistance, excellent pigment wetting and good flow. It shows good adhesion to substrates like Cellulose Acetate, Polyester, Polystyrene or Metalized Films. HVB-0001 is suitable for food packaging applications. Wash Primers: HVB-0001 can be used for formulation of wash primers for steel storage tanks, bottom of ships, airplane, bridges, dam locks, trailers, farm equipment, automotive, rail coaches etc. The material offers flexible films with good adhesion to metal surfaces. Adhesives: HVB-0001 acts as a plasticizer for adhesive formulations based on Epoxy, Phenolic, Melamine-Formaldehyde Resins and Isocyanates. It helps improving interfacial adhesion, flexibility, impact strength and resistance against solvent, chemicals or high temperature. Ceramics: HVB-0001 solution can be used as a temporary binder for ceramic powder. Typically 2 – 4 % PVB-resin are added to Ceramic. During sintering process HVB-0001 undergoes thermal degradation leaving virtually no residue. It leads to very good green strength and dimensional stability.