De Laval Nozzle - Wikipedia, the Free Encyclopedia 頁 1 / 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Che 253M Experiment No. 2 COMPRESSIBLE GAS FLOW
Rev. 8/15 AD/GW ChE 253M Experiment No. 2 COMPRESSIBLE GAS FLOW The objective of this experiment is to familiarize the student with methods for measurement of compressible gas flow and to study gas flow under subsonic and supersonic flow conditions. The experiment is divided into three distinct parts: (1) Calibration and determination of the critical pressure ratio for a critical flow nozzle under supersonic flow conditions (2) Calculation of the discharge coefficient and Reynolds number for an orifice under subsonic (non- choked) flow conditions and (3) Determination of the orifice constants and mass discharge from a pressurized tank in a dynamic bleed down experiment under (choked) flow conditions. The experimental set up consists of a 100 psig air source branched into two manifolds: the first used for parts (1) and (2) and the second for part (3). The first manifold contains a critical flow nozzle, a NIST-calibrated in-line digital mass flow meter, and an orifice meter, all connected in series with copper piping. The second manifold contains a strain-gauge pressure transducer and a stainless steel tank, which can be pressurized and subsequently bled via a number of attached orifices. A number of NIST-calibrated digital hand held manometers are also used for measuring pressure in all 3 parts of this experiment. Assorted pressure regulators, manual valves, and pressure gauges are present on both manifolds and you are expected to familiarize yourself with the process flow, and know how to operate them to carry out the experiment. A process flow diagram plus handouts outlining the theory of operation of these devices are attached. -

Rocket Nozzles: 75 Years of Research and Development
Sådhanå Ó (2021) 46:76 Indian Academy of Sciences https://doi.org/10.1007/s12046-021-01584-6Sadhana(0123456789().,-volV)FT3](0123456789().,-volV) Rocket nozzles: 75 years of research and development SHIVANG KHARE1 and UJJWAL K SAHA2,* 1 Department of Energy and Process Engineering, Norwegian University of Science and Technology, 7491 Trondheim, Norway 2 Department of Mechanical Engineering, Indian Institute of Technology Guwahati, Guwahati 781039, India e-mail: [email protected]; [email protected] MS received 28 August 2020; revised 20 December 2020; accepted 28 January 2021 Abstract. The nozzle forms a large segment of the rocket engine structure, and as a whole, the performance of a rocket largely depends upon its aerodynamic design. The principal parameters in this context are the shape of the nozzle contour and the nozzle area expansion ratio. A careful shaping of the nozzle contour can lead to a high gain in its performance. As a consequence of intensive research, the design and the shape of rocket nozzles have undergone a series of development over the last several decades. The notable among them are conical, bell, plug, expansion-deflection and dual bell nozzles, besides the recently developed multi nozzle grid. However, to the best of authors’ knowledge, no article has reviewed the entire group of nozzles in a systematic and comprehensive manner. This paper aims to review and bring all such development in one single frame. The article mainly focuses on the aerodynamic aspects of all the rocket nozzles developed till date and summarizes the major findings covering their design, development, utilization, benefits and limitations. -

Session 15 FLOWS THROUGH NOZZLE Outline
Session 15 FLOWS THROUGH NOZZLE Outline • Definition • Types of nozzle • Flow analysis • Ideal Gas Relationship • Mach Number and Speed of Sound • Isentropic flow of an ideal gas • De Laval nozzle • Nozzle efficiency • Calculation examples Definition Nozzle is a duct by flowing through which the velocity of a fluid increases at the expense of pressure drop. A duct which decreases the velocity of a fluid and causes a corresponding increase in pressure is called a diffuser Types of Nozzles There are three types of nozzles a. Convergent nozzle b. Divergent nozzle c. Convergent-divergent nozzle Flow Analysis • Incompressible flow • Compressible Flow The variation of fluid density for compressible flows requires attention to density and other fluid property relationships. The fluid equation of state, often unimportant for incompressible flows, is vital in the analysis of compressible flows. Also, temperature variations for compressible flows are usually significant and thus the energy equation is important. Curious phenomena can occur with compressible flows. Flow Analysis • For simplicity, the gas is assumed to be an ideal gas. • The gas flow is isentropic. • The gas flow is constant • The gas flow is along a straight line from gas inlet to exhaust gas exit • The gas flow behavior is compressible Ideal Gas Relationship The equation of state for an ideal gas is: For an ideal gas, internal energy, û is considered to be a function of temperature only Thus, Ideal Gas Relationship The fluid property enthalpy, ĥ is defined as Since for an ideal gas, enthalpy is a function of temperature only, the ideal gas specific heat at constant pressure, Cp can be expressed as And, Ideal Gas Relationship We see that changes in internal energy and enthalpy are related to changes in temperature by values of Cp and Cv. -

Nozzle Aerodynamics Baseline Design
Preliminary Design Review Supersonic Air-Breathing Redesigned Engine Nozzle Customer: Air Force Research Lab Advisor: Brian Argrow Team Members: Corrina Briggs, Jared Cuteri, Tucker Emmett, Alexander Muller, Jack Oblack, Andrew Quinn, Andrew Sanchez, Grant Vincent, Nathaniel Voth Project Description Model, manufacture, and verify an integrated nozzle capable of accelerating subsonic exhaust to supersonic exhaust produced from a P90-RXi JetCat engine for increased thrust and efficiency from its stock configuration. Stock Nozzle Vs. Supersonic Nozzle Inlet Compressor Combustor Turbine Project Baseline Nozzle Nozzle Test Nozzle Project Description Design Aerodynamics Bed Integration Summary Objectives/Requirements •FR 1: The Nozzle Shall accelerate the flow from subsonic to supersonic conditions. •FR 2: The Nozzle shall not decrease the Thrust-to-Weight Ratio. •FR 3: The Nozzle shall be designed and manufactured such that it will integrate with the JetCat Engine. •DR 3.1: The Nozzle shall be manufactured using additive manufacturing. •DR 3.4: Successful integration of the nozzle shall be reversible such that the engine is operable in its stock configuration after the new nozzle has been attached, tested, and detached. •FR4: The Nozzle shall be able to withstand engine operation for at least 30 seconds. Project Baseline Nozzle Nozzle Test Nozzle Project Description Design Aerodynamics Bed Integration Summary Concept of Operations JetCat P90-SE Subsonic Supersonic Engine Flow Flow 1. Remove Stock Nozzle 2. Additive Manufactured 3-D Nozzle -
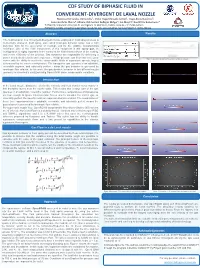
Cdf Study of Biphasic Fluid in Convergent-Divergent De
CDF STUDY OF BIPHASIC FLUID IN CONVERGENT-DIVERGENT DE LAVAL NOZZLE Maricruz Hernández-Hernández1, Victor Hugo Mercado-Lemus1, Hugo Arcos Gutiérrez2, Isaías Garduño-Olvera2, Adriana Del Carmen Gallegos Melgar1, Jan Mayen2, Raul Pérez Bustamante1. 1CONACYT, Corporación Mexicana de Investigación en Materiales, Saltillo, Coahuila, C.P. 25290, México. 2CONACYT, CIATEQ, Unidad San Luis Potosí, Eje 126 No. 225, Zona Industrial, San Luis Potosí C.P. 78395, México. Abstract Results The fluid behavior in a convergent-divergent nozzle employed in Cold spray process is numerically analyzed. Cold spray, also called Cold gas dynamic spray, has a high potential, both for the generation of coatings and for the additive manufacturing technique. One of the main components of this equipment is the spray gun, its configuration is highly important in the control of the final characteristics of the coating, and in the efficiency of the process. Gas dynamics are responsible for delivering a power at a desired velocity and temperature. A high-pressure gas flows into a de Laval Figure 1:Geometry domain and boundary conditions of nozzle. nozzle with the ability to accelerate compressible fluids at supersonic speeds, largely determined by the nozzle configuration. The transporter gas operates in an adiabatic, reversible regimen, and calorically perfect - mean the gas behavior is governed by isentropic flow relation. In this work, the gas dynamic behavior in two different nozzle geometry is numerically analyzed using OpenFOAM under compressible conditions. Introduction In the Laval nozzle, dissipative effects like viscosity and heat transfer occur mainly in thin boundary layers near the nozzle walls. This means that a large part of the gas operates in an adiabatic, reversible regimen. -

Supersonic Air and Wet Steam Jet Using Simplified De Laval Nozzle
Proceedings of the International Conference on Power Engineering-15 (ICOPE-15) November 30- December 4, 2015, Yokohama, Japan Paper ID: ICOPE-15-1158 Supersonic air and wet steam jet using simplified de Laval nozzle Takumi Komori*, Masahiro Miura*, Sachiyo Horiki* and Masahiro OSAKABE* * Tokyo University of Marine Science & Technology 2-1-6Etchujima, Koto-ku, Tokyo 135-8533, Japan E-mail: [email protected] Abstract Usually, Trichloroethane has been used for the de-oiling and cleaning of machine parts. But its production and import have been prohibited since 1995 because of its possibility to destroy the ozone layer. Generally for biological and environmental safety, the de-oiling should be done with the physical method instead of the chemical method using detergent or solvent. As one of the physical method, a cleaning by a supersonic wet steam jet has been proposed. The steam jet with water droplets impinges on the oily surface of machine parts and removes the oil or smudge. In the present study, the low-cost and taper-shaped nozzles were fabricated with an electric discharge machining. The jet behaviors from the taper-shaped nozzles were carefully observed by using air and wet steam. The non-equilibrium model of wet steam was proposed and compared with the experimental results. The spatial distribution of low density regions along the jet axis was considered to contribute the cleaning and de-oiling. However, the condensate generated with the depressurization of steam depressed the injected steam mass flow rate. Furthermore, the steam cleaning was conducted for the plastic coin and the good cleaning effect could be confirmed in spite of the short cleaning duration. -

Flow Analysis of Rocket Nozzle Using Method of Characteristics
FLOW ANALYSIS OF ROCKET NOZZLE USING METHOD OF CHARACTERISTICS Dipen R. Dangi1, Parth B. Thaker2, Atal B. Harichandan3 1, 2, 3 Department of Mechanical Engineering, Marwadi Education Foundation, (India) ABSTRACT The present research work has been carried out for the analysis of bell type nozzle by using method of characteristic with different Mach number conditions. A C-code has been developed to define the nozzle geometry by using method of characteristics usually designed for De-Laval nozzle. ANSYS 14.0 has been used for the present flow analysis by considering hear Stress Transport k-ω (SSTKW) turbulence model. In this paper CFD is used to develop best geometry of rocket nozzle with consider of aero - thermodynamic property like pressure, velocity, temperature and Mach number. Keywords: Design Of Supersonic Nozzle, Method Of Characteristic And Minimum Length Of Nozzle. I INTRODUCTION Performance of rocket nozzle is always less than the theoretical approach in all practical approaches. It is always essential to improve the performance of rocket nozzle. Previous research works show the De- Laval nozzle is the most efficient nozzle among all other types of nozzles. The De-Laval nozzle gives the best performance rocket nozzle as Mach number and velocity of fluid flow is continuously increasing while at same time pressure is continuously decreasing. But still some research gap is there to develop best geometry of rocket nozzle. Even today most of the engineers are work on how to develop best geometry of rocket nozzle and to achieve higher Mach number and higher velocity of fluid flow. Due to that reason authors works on the Method of Characteristics (MOC) and developed best geometry which gives possible optimum values of Mach number, velocity, pressure distribution and density distribution. -

Rocket Nozzles the Simplest Nozzle Is a Cone with a Half-Opening Angle, Α Attached to a Combustion Chamber (See Figure 3)
NOZZLES S. R. KULKARNI 1. Motivation In astronomy textbooks it is usually stated that the Bondi-Hoyle solution has a specific value for the accretion rate (given the boundary conditions of ambient density and temper- ature of the gas). Only with this specific accretion rate will there be a seamless transition from sub-sonic inflow to super-sonic inflow. The Bondi-Hoyle or the Parker problem is quite analogous to the rocket problem, in that both involve a smooth transition from sub-sonic to super-sonic flow. What bothered me is the statement in astronomy books that the solution demands a specific accretion (or excretion) mass rate. In contrast, the thrust on a rocket can be varied (as can be gathered if you watch NASA TV). Given the similarity between the astronomical problem and the rocket problem I thought an investigation of the latter may be illuminating and hence this note. 2. The de Laval Nozzle Consider a rocket which is burning fuel. Let M˙ be the burn rate of the fuel and ue be the speed of the exhaust. Then in steady state the vertical “thrust” or force is Mu˙ e. The resulting vertical thrust lifts the rocket. Clearly, it is of greatest advantage to make the exhaust speed as large as possible and to minimize the outflow in directions other than vertical. A rocket engine consists of a chamber in which fuel is burnt connected to a nozzle. A properly designed1 nozzle can convert the hot burnt fuel into supersonic flow (see Figure 1). The integration of the momentum equation (under assumptions of inviscid flow) yields the much celebrated Beronulli’s theorem. -

Characterization of Non-Equilibrium Condensation of Supercritical Carbon Dioxide in a De Laval Nozzle
Characterization of Non-Equilibrium Condensation of Supercritical Carbon Dioxide in a de Laval Nozzle The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation Lettieri, Claudio, Derek Paxson, Zoltan Spakovszky, and Peter Bryanston-Cross. “Characterization of Non-Equilibrium Condensation of Supercritical Carbon Dioxide in a de Laval Nozzle.” Volume 9: Oil and Gas Applications; Supercritical CO2 Power Cycles; Wind Energy (June 26, 2017). As Published http://dx.doi.org/10.1115/GT2017-64641 Publisher ASME International Version Final published version Citable link http://hdl.handle.net/1721.1/116087 Terms of Use Article is made available in accordance with the publisher's policy and may be subject to US copyright law. Please refer to the publisher's site for terms of use. Proceedings of ASME Turbo Expo 2017: Turbomachinery Technical Conference and Exposition GT2017 June 26-30, 2017, Charlotte, NC, USA GT2017-64641 CHARACTERIZATION OF NON-EQUILIBRIUM CONDENSATION OF SUPERCRITICAL CARBON DIOXIDE IN A DE LAVAL NOZZLE Claudio Lettieri Derek Paxson Delft University of Technology Massachusetts Institute of Technology Delft, The Netherlands Cambridge, MA, USA Zoltan Spakovszky Peter Bryanston-Cross Massachusetts Institute of Technology Warwick University Cambridge, MA, USA Coventry, UK ABSTRACT data, with improved accuracy at conditions away from the On a ten-year timescale, Carbon Capture and Storage could critical point. The results are applied in a pre-production significantly reduce carbon dioxide (CO2) emissions. One of supercritical carbon dioxide compressor and are used to define the major limitations of this technology is the energy penalty inlet conditions at reduced temperature but free of for the compression of CO2 to supercritical conditions, which condensation. -

Acoustic and Aerodynamic Characteristics Of
Aeroacoustic Characteristics of Model Jet Test Facility Flow Conditioners Kevin W. Kinzie*, Brenda S. Henderson**, and Harry H. Haskin*** NASA Langley Research Center, Hampton , VA 23681 An experimental investigation of flow conditioning devices used to suppress internal rig noise in high speed, high temperature experimental jet facilities is discussed. The aerodynamic and acoustic characteristics of a number of devices including pressure loss and extraneous noise generation are measured. Both aerodynamic and acoustic characteristics are strongly dependent on the porosity of the flow conditioner and the closure ratio of the duct system. For unchoked flow conditioners, the pressure loss follows conventional incompressible flow models. However, for choked flow conditioners, a compressible flow model where the duct and flow conditioner system is modeled as a convergent-divergent nozzle can be used to estimate pressure loss. Choked flow conditioners generate significantly more noise than unchoked conditioners. In addition, flow conditioners with small hole diameters or sintered metal felt material generate less “self-noise” noise compared to flow conditioners with larger holes. Nomenclature AR = duct closure ratio CB = counterbored flow conditioner d1 = flow conditioner minimum hole diameter d2 = flow conditioner counterbore hole diameter FCPR = flow conditioner pressure ratio OPR = operating pressure ratio POA = percent open area; porosity l1 = flow conditioner counterbore depth L = flow conditioner thickness mic = flow conditioner with microholes K = pressure loss coefficient st = flow conditioner with straight through holes; no counterbore StD = Strouhal number based on exit orifice diameter and velocity Stfc = Strouhal number based on flow condition hole diameter and velocity U = flow velocity θ = directivity angle ρ = density I. -

Module 13: Critical Flow Phenomenon Joseph S. Miller, PE
Fundamentals of Nuclear Engineering Module 13: Critical Flow Phenomenon Joseph S. Miller, P.E. 1 2 Objectives: Previous Lectures described single and two-phase fluid flow in various systems. This lecture: 1. Describe Critical Flow – What is it 2. Describe Single Phase Critical Flow 3. Describe Two-Phase Critical Flow 4. Describe Situations Where Critical Flow is Important 5. Describe origins and use of Some Critical Flow Correlations 6. Describe Some Testing that has been Performed for break flow and system performance 3 Critical Flow Phenomenon 4 1. What is Critical Flow? • Envision 2 volumes at different pressures suddenly connected • Critical flow (“choked flow”) involves situation where fluid moves from higher pressure volume at speed limited only by speed of sound for fluid – such as LOCA • Various models exist to describe this limiting flow rate: • One-phase vapor, one phase liquid, subcooled flashing liquid, saturated flashing liquid, and two-phase conditions 5 2. Critical Single Phase Flow Three Examples will be given 1.Steam Flow 2.Ideal Gas 3.Incompressible Liquid 6 Critical Single Phase Flow - Steam • In single phase flow: sonic velocity a and critical mass flow are directly related: g v(P,T)2 a2 = c dv(P,T) dP S 2 g G = a2 ρ(P,T )2 = c crit dv(P,T ) dP S • Derivative term is total derivative of specific volume evaluated at constant entropy • Tabulated values of critical steam flow can be found in steam tables 7 Example Critical Steam Flow Calculation • Assume 2 in2 steam relief valve opens at 1000 psi • What is steam mass discharge rate? • Assume saturated system with Tsat = 544.61°F • f = 50.3 • Wcrit = f P A =(50.3lb-m/hr)(1000 psi)(2 in2) = 100,600 lb-m/hr = 27.94 lb-m/sec. -

Performance Evaluation of an IC Engine in the Presence of a C-D Nozzle in the Air Intake Manifold
International Journal of Current Engineering and Technology ISSN 2277 - 4106 © 2013 INPRESSCO. All Rights Reserved. Available at http://inpressco.com/category/ijcet Research Article Performance Evaluation of an IC Engine in the Presence of a C-D Nozzle in the Air Intake Manifold S.Ravi Babu*a, K.Prasada Raoa and P.Ramesh Babua aDepartment of Mechanical Engineering, GMR Institute of Technology, Rajam, India Accepted 10 August 2013, Available online 01 October 2013, Vol.3, No.4 (October 2013) Abstract The present day energy crisis and ever increasing demands of energy in addition to global pollution brought us into a situation where there is an urgent need for energy conservation, efficient utilization and eco-friendly techniques to be implemented in day to day use. These needs lead us to an idea of modified design in a CI engine without any additional energy requirement and with no complicated variations in design. There are various other methods to improve the efficiency of engine such as super charging, turbo charging, varying stroke length, varying injection pressure, fuel to air ratio, additional strokes per cycle and so on. Many of them require additional design (stroke length, injection pressure etc.,) and some of them load to increase environmental effect. Here in this project affords were made to increase the velocity (physical parameter) of air entering the inlet manifold of the engine by inserting a convergent divergent nozzle at the inlet manifold. There by increasing the mixture quality of air & fuel in the combustion chamber before the initialization of ignition. The engine load tests were carried out at different loads, variation of different parameters with load was plotted.