Kinetics and Modeling of Reductive Dechlorination at High PCE and TCE Concentrations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chlorinated Solvent Bioremediation: Fundamentals and Practical Application for Remedial Project Managers Presented By: Christopher Marks, Ph.D
Chlorinated Solvent Bioremediation: Fundamentals and Practical Application for Remedial Project Managers Presented by: Christopher Marks, Ph.D. & Carolyn Acheson, Ph.D. US Environmental Protection Agency Office of Research and Development National Risk Management Research Laboratory CLU-IN Webinar Nov. 14, 2018 Source: Accelerated Bioremediation of Chlorinated Solvents, Interstate Technology and Regulatory Council and Remediation and Technology Development Forum, 2003 1 Bioremediation of Chlorinated Solvents Source: Accelerated Bioremediation of Chlorinated Solvents, Interstate Technology and Regulatory Council and Remediation and Technology 2 Development Forum, 2003 Part I: Introduction to Chlorinated Solvent Properties and Anaerobic Reductive Dechlorination 3 Terminology • Anaerobic: Microbial metabolic processes occurring in the absence of oxygen. • Anaerobic Reductive Dechlorination: The biological removal of a chlorine atom from an organic compound and replacement with a hydrogen atom in a reducing environment. • Biodegradation aka biotransformation: Biologically mediated reactions which convert one chemical to another. For example, PCE is converted to TCE when anaerobic reductive reactions remove a chlorine molecule. • Bioremediation: The engineered approaches using microorganisms to biodegrade contaminants. • Biostimulation: The addition of organic electron donors and nutrients to enhance the rate of reductive dechlorination by the native microflora. • Bioaugmentation: The addition of beneficial microorganisms to enhance the capacity -

Overview of in Situ Bioremediation of Chlorinated Ethene DNAPL Source Zones
INTERSTATE TECHNOLOGY & REGULATORY COUNCIL Warning! This document has not been amended since publication. Some content may be out of date and may no longer apply. INTERSTATE TECHNOLOGY & REGULATORY COUNCIL Technology Overview Overview of In Situ Bioremediation of Chlorinated Ethene DNAPL Source Zones October 2005 Prepared by The Interstate Technology & Regulatory Council Bioremediation of DNAPLs Team ABOUT ITRC Established in 1995, the Interstate Technology & Regulatory Council (ITRC) is a state-led, national coalition of personnel from the environmental regulatory agencies of more than 40 states and the District of Columbia, three federal agencies, tribes, and public and industry stakeholders. The organization is devoted to reducing barriers to and speeding interstate deployment of, better, more cost-effective, innovative environmental techniques. ITRC operates as a committee of the Environmental Research Institute of the States (ERIS), a Section 501(c)(3) public charity that supports the Environmental Council of the States (ECOS) through its educational and research activities aimed at improving the environment in the United States and providing a forum for state environmental policy makers. More information about ITRC and its available products and services can be found on the Internet at www.itrcweb.org. DISCLAIMER This document is designed to help regulators and others develop a consistent approach to their evaluation, regulatory approval, and deployment of specific technologies at specific sites. Although the information in this document is believed to be reliable and accurate, this document and all material set forth herein are provided without warranties of any kind, either express or implied, including but not limited to warranties of the accuracy or completeness of information contained in the document. -

Considerations for the Collection and Evaluation of Natural Attenuation Parameters at Sites Contaminated with Chlorinated Solvents
CONSIDERATIONS FOR THE COLLECTION AND EVALUATION OF NATURAL ATTENUATION PARAMETERS AT SITES CONTAMINATED WITH CHLORINATED SOLVENTS Chlorine • Hydrogen • Carbon • Bond "\. ( . I Vinyl Chloride I . ~ I Cis-1,2-Dichloroethene I ITrichloroethene I .,. Source Area Biological Active Area DowngradientI Area Filure 3 Anaerobic Reductive Dechlorination ofa Trichloroethene Plnm.e Prepared by Florida Department of Environmental Protection (FDEP) Division of Waste Management Bureau of Waste Cleanup Hazardous Waste Cleanup Section April 1999 TABLE OF CONTENTS SECTION TITLE PAGE 1.0 IN'TR.ODUCTION ........................................................................................ 1 ~ 2.0 GENERAL OVERVIEW OF CHLORINATED ALIPHATIC HYDROCARBON BIODEGRADATION................................................. 2 2.1 Bacteria 2.2 Electron Acceptor Reactions 2.3 Electron Donor Reactions 2.4 Co-Metabolism 3.0 NATURAL ATTENUATION BY PHYSICAL PROCESSES ................... 4 4.0 METABOLIC BY-PRODUCTS AND REDOX PROCESSES IN GROUNDWATER SYSTEMS ................................................................... 4 S.O EVIDENCE USED TO SUPPORT NATURAL ATTENUATION•••••••••• S 5.1 Direct Evidence S.2 Examine Changes 5.3 Laboratory microcosm studies 6.0 METHODOLOGY USED FOR DATA REVIEW AND SITE EVALUATION........................................................................................... 6 7.0 NATURAL ATTENUATION SAMPLING AND DATA EVALUATION METHODOLOGY..................................................................................... 9 7.1 Monitor Well -
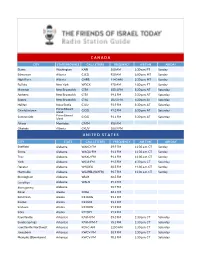
C a N a D a U N I T E D S T a T
C A N A D A CITY STATE/PROVINCE CALL LETTERS FREQUENCY AIR TIME AIR DAY Blaine Washington KARI 550 AM 1:30 a.m. PT Sunday Edmonton Alberta CJCD 930 AM 6:00 p.m. MT Sunday High River Alberta CHRB 1140 AM 2:30 p.m. MT Sunday Buffalo New York WDCX 970 AM 1:00 p.m. ET Sunday Moncton New Brunswick CITA 105.1 FM 5:30 p.m. AT Saturday Amherst New Brunswick CITA 99.1 FM 5:30 p.m. AT Saturday Sussex New Brunswick CITA 107.3 FM 5:30 p.m. AT Saturday Halifax Nova Scotia CJLU 93.9 FM 5:30 p.m. AT Saturday Charlottetown Prince Edward CIOG 91.3 FM 5:30 p.m. AT Saturday Island Summerside Prince Edward CIOG 91.1 FM 5:30 p.m. AT Saturday Island Altona Manitoba CFAM 950 AM Okotoks Alberta CKUV 100.9 FM U N I T E D S T A T E S CITY STATE CALL LETTERS FREQUENCY AIR TIME AIR DAY Sheffield Alabama WAKD-FM 89.9 FM 11:30 a.m. CT Sunday Selma Alabama WAQU-FM 91.1 FM 11:30 a.m. CT Sunday Troy Alabama WAXU-FM 91.1 FM 11:30 a.m. CT Sunday York Alabama WSJA-FM 91.3 FM 4:30 p.m. CT Saturday Decatur Alabama W203DJ 88.5 FM 11:30 a.m. CT Sunday Huntsville Alabama W229BL (WAFR) 93.7 FM 11:30 a.m. CT Sunday Birmingham Alabama WLJR 88.5 FM Carrollton Alabama WALN 89.3 FM Montgomery Alabama 92.7 FM Kenai Alaska KOGJ 88.1 FM Ketchikan Alaska K216DG 91.1 FM Kodiak Alaska K216DF 91.1 FM Seldovia Alaska K220FW 91.9 FM Sitka Alaska K220FY 91.9 FM Fayetteville Arkansas KAYH-FM 89.3 FM 1:30 p.m. -

Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents
FINAL Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents August 2004 This page intentionally left blank PRINCIPLES AND PRACTICES OF ENHANCED ANAEROBIC BIOREMEDIATION OF CHLORINATED SOLVENTS August 2004 Prepared for: Air Force Center for Environmental Excellence Brooks City-Base, Texas Naval Facilities Engineering Service Center Port Hueneme, California and Environmental Security Technology Certification Program Arlington, Virginia This page intentionally left blank ACKNOWLEDGEMENTS The Parsons Corporation (Parsons) prepared this Principles and Practices document under contract to the Air Force Center for Environmental Excellence (AFCEE, Contract F41624-00- D-8024) and the Naval Facilities Engineering Service Center (NFESC, Contract N47408-98- D-7527). The NFESC contract was funded by the Environmental Security Technology Certification Program (ESTCP). The United States Army Corp of Engineers (USACE) assisted with technical review. This document is intended to assist AFCEE, NFESC, ESTCP, USACE and their United States (US) Department of Defense (DoD) technology-transition partners in evaluating and applying enhanced in situ anaerobic bioremediation for restoration of groundwater contaminated with chlorinated solvents. The authors acknowledge the assistance of numerous individuals who provided review services, and to several environmental contractors that provided case studies and information regarding respective areas of expertise. These individuals and their affiliations are listed in Appendix A. - i - 022/738863/28.doc DISCLAIMER In no event shall either the United States Government or Parsons have any responsibility or liability for any consequences of any use, misuse, inability to use, or reliance on the information contained herein; nor does either warrant or otherwise represent in any way the accuracy, adequacy, efficacy, or applicability of the contents hereof. -

Biochemistry and Physiology of Halorespiration by Desulfitobacterium Dehalogenans
Biochemistry and Physiology of Halorespiration by Desulfitobacterium dehalogenans ..?.^TJ?*LE_ LANDBOUWCATALOGU S 0000 0807 1728 Promotor: Dr.W.M . deVo s hoogleraar in de microbiologie Co-promotoren: Dr.ir .A.J.M .Stam s universitair hoofddocent bij deleerstoelgroe p Microbiologie Dr.ir . G. Schraa universitair docent bij deleerstoelgroe p Microbiologie Stellingen 1. Halorespiratie is een weinig efficiente wijze van ademhalen. Dit proefschrift 2. Halorespiratie moet worden opgevat als verbreding en niet als specialisatie van het genus Desulfitobacterium. Dit proefschrift 3. Reductieve dehalogenases zijn geen nieuwe enzymen. 4. 16S-rRNA probes zijn minder geschikt voor het aantonen van specifieke metabole activiteiten in een complex ecosysteem. Loffler etal. (2000) AEM66 : 1369;Gottscha l &Kroonema n (2000)Bode m3 : 102 5. Het "twin-arginine" transportsysteem wordt niet goed genoeg begrepen om op basis van het voorkomen van het "twin-arginine" motief enzymen te lokaliseren. Berks etal. (2000)Mol .Microbiol .35 : 260 6. Asbesthoudende bodem is niet verontreinigd. 7. Biologische groente is een pleonasme. Stellingen behorende bij het proefschrift 'Biochemistry and physiology of halorespiration by Desulfitobacterium dehalogenans' van Bram A. van de Pas Wageningen, 6 december 2000 MJOQ^O \lZ°]0 ^ Biochemistry and Physiology of Halorespiration by Desulfitobacterium dehalogenans BramA. van de Pas Proefschrift ter verkrijging van de graad van doctor op gezag van derecto r magnificus van Wageningen Universiteit, dr. ir. L. Speelman, in het openbaar -

The Influence of In-Situ Activated Carbon on Biodegradation of Chlorinated Solvents
Clemson University TigerPrints All Theses Theses 8-2018 The nflueI nce of In-Situ Activated Carbon on Biodegradation of Chlorinated Solvents Kameryn McGee Clemson University, [email protected] Follow this and additional works at: https://tigerprints.clemson.edu/all_theses Recommended Citation McGee, Kameryn, "The nflueI nce of In-Situ Activated Carbon on Biodegradation of Chlorinated Solvents" (2018). All Theses. 2925. https://tigerprints.clemson.edu/all_theses/2925 This Thesis is brought to you for free and open access by the Theses at TigerPrints. It has been accepted for inclusion in All Theses by an authorized administrator of TigerPrints. For more information, please contact [email protected]. THE INFLUENCE OF IN-SITU ACTIVATED CARBON ON BIODEGRADATION OF CHLORINATED SOLVENTS A Thesis Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Master of Science Environmental Engineering and Earth Sciences by Kameryn McGee August 2018 Accepted by: Dr. Kevin Finneran, Committee Chair Dr. David Ladner Dr. Sudeep Popat ABSTRACT Chlorinated solvents have been a contaminant of interest in the remediation field for many years because they have been manufactured in large amounts and released into the environment due to improper storage and disposal. Since they are produced in such large quantities and there has been so many cases of uncontrolled releases, corrective actions are necessary and many remediation strategies have been explored. In- situ bioremediation of chlorinated solvents (TCE) is an intricate respiratory process which combines the addition of electron donors, chemical reactions, and the presence of microorganisms which directly access contaminants. Microbes metabolize the electron donor and utilize the energy for complete dechlorination of TCE to ethene. -

Family Handbook
FamilyHandbook Contact Information: Amber Palicke Coordinator (810) 794-9317 option 1305 Angie Berube Lead Teacher (810) 794-8876 option 5 Nichole Brody Associate Teacher Dana Bosel Lead Teacher (810) 794-8876 option 4 Melissa Mann Associate Teacher Lisa MerloTransportation (810) 794-3555 Alan Latosz Superintendent (810) 794-9364 “These materials were developed under a grant awarded by the Michigan Department of Education” 1 Table of Contents o Eligibility & Enrollment 3 o RecruitmentPolicy 3 o Referral Policy 3 o Sliding Fee Tuition Scale Policy 4 o Program Philosophy 5 o Curriculum, Screening &Assessment 5-6 o Daily Schedule 6-8 o Transportation 8 o Attendance Policy 8-9 o Exclusion/Health Policy 9-10 o Medication Policy 10 o Dismissal Procedures 10 o Nutrition Policy 11 o Physical Activity Policy 11 o Rest Policy 11 o Clothing Policy 11-12 o Weather Policy 12 o Discipline Policy 12 o Accident/Injury Policy 12 o Emergency Policy 12-13 o Child Abuse/Neglect 13 o Confidentiality 13 o Grievances 13 o Parent Involvement 13-15 o Notice of Program Measurement 15 o Licensing 15 2 o School Calendar 16 Eligibility & Enrollment: The Great Start Readiness Program (GSRP) is a free preschool that prepares children for kindergarten. In order to be eligible for GSRP, students must be four years of age on or before September first of the current school year and meet income and risk factor requirements. Applications can be accessed online at www.acsk12.us and in the community at Pointe Tremble Early Childhood Center at 9541 Phelps Road in Clay Township.Completed applications and accompanying documents should be returned to GSRP staff at Pointe Tremble Early Childhood Center.Required accompanying documents include proof of family income, documentation of child’s date of birth, completed health appraisal signed by a physician, immunization record, completed child information record, volunteer ICHAT form, and a completed application for free and reduced price lunch. -

The Southern California Radio Reference Guide 4/29/2020
The Southern California Radio Reference Guide 4/29/2020 Call letters Branding Dial position Ownership Nielsen Market Format Phone Website KATY 101.3fm The Mix 101.3 FM All Pro Broadcasting Riverside/San Bernardino Adult Contemporary (951) 506-1222 http://www.1013themix.com/ KHTI Hot 103.9 103.9 FM All Pro Broadcasting Riverside/San Bernardino Hot AC (909) 890-5904 http://www.x1039.com/ KKBB Groove 99-3 99.3 FM Alpha Media USA Bakersfield Rhythmic Oldies (661) 393-1900 https://www.groove993.com/ KLLY Energy 95.3 95.3 FM Alpha Media USA Bakersfield Hot AC (661) 393-1900 https://www.energy953.com/ KNZR 1560 & 97.7 FM KNZR 1560 AM Alpha Media USA Bakersfield News Talk (661) 393-1900 https://www.knzr.com/ KCLB 93.7 KCLB 93.7 FM Alphamedia Palm Springs Rock (760) 322-7890 https://www.937kclb.com/ KDES 98.5 The Bull 98.5 FM Alphamedia Palm Springs Country (760) 322-7891 https://www.985thebull.com/ KDGL The Eagle 106.9 106.9 FM Alphamedia Palm Springs Classic Rock (760) 322-7890 https://www.theeagle1069.com/ U-92.7 The Desert's KKUU 92.7 FM Alphamedia Palm Springs Dance CHR (760) 322-7890 https://www.u927.com/ Hottest Music KNWH / KNWQ / KNWZ K-News, The Voice of 1250 AM/1140 AM/970 Alphamedia Palm Springs Talk (760) 322-7890 https://www.knewsradio.com/ AM & FM The Valley AM/94.3 FM Mix 100.5 The Desert's KPSI FM 100.5 Alphamedia Palm Springs Hot AC (760) 322-7890 https://www.mix1005.fm/ Best Mix KCAL 96.7 K-CAL Rocks 96.7 FM Anaheim Broadcasting Corporation Riverside/San Bernardino Rock (909) 793-3554 https://www.kcalfm.com/ KOLA KOLA 99.9 99.9 FM Anaheim Broadcasting Corporation Riverside/San Bernardino Oldies (909) 793-3554 https://www.kolafm.com/ KCWR Real Country 107.1 FM Buck Owens Broadcasting Bakersfield Country (661) 326-1011 N/A KRJK 97.3 The Bull 97.3 FM Buck Owens Broadcasting Bakersfield Adult HIts (661) 326-1011 https://www.bull973.com/ KUZZ AM/FM (simulcast) KUZZ AM 55 ▪ FM 107.9 550 AM/107.9 FM Buck Owens Broadcasting Bakersfield Country (661) 326-1011 http://www.kuzzradio.com/ KWVE FM K-Wave 107.9 FM Calvary Chapel Church, Inc. -

NATIONAL SHOWS – LIVE and INTERACTIVE – COAST to COAST New York • Los Angeles • Chicago • San Francisco • Philadelphia • Phoenix • +150 More!
NATIONAL SHOWS – LIVE AND INTERACTIVE – COAST TO COAST New York • Los Angeles • Chicago • San Francisco • Philadelphia • Phoenix • +150 more! Ashley Noronha Rome Correspondent Msgr. Stuart Swetland Chief Religion Correspondent JOIN THE CONVERSATION! Call our studio line at 888-914-9149 ET CT Week Days Saturday Sunday MT PT 6a 5a Morning Air® 4a 3a 7a 6a Children’s Rosary 5a 4a Morning Air® 7:30a 6:30a Morning Air® Christopher Closeup 5:30a 4:30a 8a 7a Inspiring, informative, joyful, and family-friendly conversations to start your day. Life is Worth Living 6a 5a 9a 8a Where God Weeps 7a 6a 9:30a 8:30a Word on Fire™ 7:30a 6:30a Patrick Madrid Show 10a 9a The Patrick Madrid Show Sunday Mass 8a 7a 11a 10a Your source for the latest in current events, culture trends, and contemporary issues. The Miracle Hunter® 9a 8a noon 11a The Inner Life® Inner Life® Trending 10a 9a On air spiritual direction from a rotating panel of highly experienced Catholic priests. 1p noon Go Ask Your Father™ Go Ask Your Father™ Dan Cheely Show™ 11a 10a Answers to your questions about faith and morals, doctrine and social teaching. 2p 1p Word on Fire™ noon 11a Father Simon Says™ Father Simon Says™ 2:30p 1:30p St Paul Center Presents 12:30p 11:30a Your daily bible study with Scripture readings, Word of the Day, and faith Q&A. 3p 2p 1p noon 4p 3p The Drew Mariani Show™ Drew Mariani Show™ Drew Mariani Show™ 2p 1p 5p 4p Breaking news coverage and conversation. -

Redutive Dehalogenation of Chlorinated Alkanes by Novel Bacteria at the Petroprocessor of Louisiana Inc
Louisiana State University LSU Digital Commons LSU Doctoral Dissertations Graduate School 2008 Redutive Dehalogenation of Chlorinated Alkanes by Novel Bacteria at the PetroProcessor of Louisiana Inc. Superfund Site Jun Yan Louisiana State University and Agricultural and Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_dissertations Part of the Civil and Environmental Engineering Commons Recommended Citation Yan, Jun, "Redutive Dehalogenation of Chlorinated Alkanes by Novel Bacteria at the PetroProcessor of Louisiana Inc. Superfund Site" (2008). LSU Doctoral Dissertations. 510. https://digitalcommons.lsu.edu/gradschool_dissertations/510 This Dissertation is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Doctoral Dissertations by an authorized graduate school editor of LSU Digital Commons. For more information, please [email protected]. REDUCTIVE DEHALOGENATION OF CHLORINATED ALKANES BY NOVEL BACTERIA AT THE PETROPROCESSOR OF LOUISIANA INC. SUPERFUND SITE A Dissertation Submitted to the Graduate Faculty of the Louisiana State University and Agriculture and Mechanical College in partial fulfillment of the requirements for the degree of Doctor of Philosophy in The Department of Civil and Environmental Engineering by Jun Yan B.S., Nanjing University, China, 2000 M.S., Nanjing University, China, 2003 May 2009 ACKNOWLEDGMENTS I would like to take this opportunity to express my sincere appreciation to my advisor, Dr. William M. Moe, for offering me such a great opportunity to complete my Ph.D. research under his direction. During my study at LSU, I have enjoyed my research on this project in Dr. Moe’s group. His insightful guidance and generous support always inspire my research and encourage me to pursue the accomplishments of my career. -

Electron Donor and Acceptor Utilization by Halorespiring Bacteria'
Electron donor andaccepto r utilizationb y halorespiring bacteria Maurice L.G.C. Luijten CENTRALS LANDBOUWCATALOGUS 0000 0950 9080 Promotoren Prof.dr .W.M . de Vos Hoogleraar ind eMicrobiologi c Wageningen Universiteit Prof.dr .ir .A.J.M .Stam s Persoonlijk hoogleraar bij hetlaboratoriu mvoo r Microbiologic Laboratorium voor Microbiologic, Wageningen Universiteit Copromotoren Dr.G .Schra a Universitair docent Laboratorium voor Microbiologic, Wageningen Universiteit Dr.A.A.M . Langenhoff Senior researcher/project leader Milieubiotechnologie, TNO-MEP Promotiecommissie Prof. E.J.Bouwe r John's Hopkins University, Baltimore, USA Prof.dr .C .Hollige r EPFL, Lausanne, Switzerland Dr.F .Volkerin g Tauw bv, Deventer, Nederland Prof. dr.ir .W.H . Rulkens Wageningen Universteit Dit onderzoek is uitgevoerd binnen de onderzoekschool SENSE (Netherlands Research School forth e Socio-Economic andNatura l Sciences ofth e Environment). LV''-:- v. •:-:::'.Ifir Electron donor and acceptor utilizationb y halorespiring bacteria Maurice L.G.C.Luijte n Proefschrift Terverkrijgin g van de graadva n doctor opgeza gva n derecto r magnificus van Wageningen Universiteit, prof. dr. ir. L. Speelman, inhe t openbaar te verdedigen opvrijda g 11 juni 2004 desnamiddag s te half twee in deAula . \ '\ i ocXi ^ Electron donor and acceptor utilization byhalorespirin g bacteria Maurice L.G.C. Luijten Ph.D.thesi sWageninge n University, Wageningen, The Netherlands 2004 ISBN 90-5804-067-1 Front cover: Modified EMpictur e ofSulfurospirillum halorespirans PCE-M2 — ry ' Stellingen 1.El k nadeel heb zijn voordeel. Dit proefschrift. 2. De ene volledige reductie van PCE is de andere nog niet. Dit proefschrift. 3. Sectorale communicatie reikt niet ver genoeg. HRH the Prince of Orange, Wat.