Physics 2111 Laboratory Manual
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Design and Test of a Miniature Hydrogen Production Integrated Reactor
reactions Article Design and Test of a Miniature Hydrogen Production Integrated Reactor Ion Velasco, Oihane Sanz * , Iñigo Pérez-Miqueo, Iñigo Legorburu and Mario Montes Department Applied Chemistry, University of the Basque Country (UPV/EHU), 20018 San Sebastian, Spain; [email protected] (I.V.); [email protected] (I.P.-M.); [email protected] (I.L.); [email protected] (M.M.) * Correspondence: [email protected] Abstract: A detailed study of the experimental issues involved in the design and operation of a methanol steam microreformer is presented in this paper. Micromachining technology was utilized to fabricate a metallic microchannel block coupling the exothermic and endothermic process. The microchannel block was coated with a Pd/ZnO catalyst in the reforming channels and with Pd/Al2O3 in the combustion channels by washcoating. An experimental system had been designed and fine- tuned allowing estimation of the heat losses of the system and to compensate for them by means of electric heating cartridges. In this way, the heat necessary for the reforming reaction is provided by methanol combustion, thanks to the temperature and flow cascade controller we developed. Thus, the coupling of both reactions in a block of microchannels without the interference caused by significant heat loss due to the small size of the laboratory microreactor could be studied. Runs of this microreformer device were carried out, varying the deposited catalyst amount, methanol steam Citation: Velasco, I.; Sanz, O.; reforming temperature and space velocity. When the reforming reaction was compensated by the Pérez-Miqueo, I.; Legorburu, I.; combustion reaction and the heat losses by the electric heating, an almost isothermal behavior of the Montes, M. -

Ballistic Pendulum
PH 1113: Ballistic Pendulum Ballistic Pendulum Objective The purpose of this experiment1 is to determine the muzzle velocity of a projectile launcher, using the conservation of momentum and the conservation of energy (where applicable). Materials 1. 1-meter stick 4. Projectile (metal ball) 2. 30-cm ruler 5. Triple-beam balance 3. Ballistic pendulum machine Introduction The ballistic pendulum is a classic method for determining the velocity of a projectile; origi- nally, it was used to determine the muzzle velocity of firearms. It is also a good demonstration of many basic principles of physics. In ballistic pendula experiments a projectile (in our case a ball) is fired into a stationary, freely hanging pendulum, which swings up by a measured amount after being impacted by the projectile. From that measured angle and from the length of the pendulum, you can determine the height reached by the center of mass of the pendulum; then you can calculate its gravitational potential energy. We know that mechanical energy (ME) is conserved, so we know that the gravitational potential energy at the maximum height must be equal to the kinetic energy of the pendulum at the bottom of the swing, just after the ball collides with the pendulum. In symbolic terms, MEi = MEf ; (1) where ME = PE + KE. So, 0 0 ¨¨* ¨¨* ¨PEi + KEi = PEf + ¨KE¨f (2) 1Some of the text of this manual was taken from the Pasco Ballistic Pendulum/Projectile Launcher Instruction Manual. 1 Mississippi State University Department of Physics and Astronomy You cannot, however, equate the kinetic energy of the pendulum after the collision with the kinetic energy of the ball before the swing, because the collision between ball and pendulum is inelastic, and kinetic energy is not conserved in inelastic collisions. -

INTERMEDIATE LABORATORY - PHYX 3870-3880 List of Experiments Fall 2015 MECHANICS M1
INTERMEDIATE LABORATORY - PHYX 3870-3880 List of Experiments Fall 2015 MECHANICS M1. Kater's Pendulum A straightforward lab using a reversible physical pendulum to measure the local acceleration of gravity, g, to less than 0.01%. Emphasis is given to detailed error analysis and error propagation and to high precision measurements. Computer interfacing with photogates and motion sensors. A good beginning lab. M2. Coupled Pendulum Investigation of two pendulums coupled by a spring between them. Lab emphasizes comparison of measured results of the periods and damping to values derived from a theoretical model using differential equations. Completion of Analytical Mechanics is recommended. Computerized data collection with voltage probes and motion sensors and data reduction are used. ELECTRICITY & MAGNETISM E2. Thompson's e/m Experiment Measures the ratio of the charge to mass of an electron (e/m) by investigating the trajectory of electrons in electric and magnetic fields. The British physicist J. J. Thompson received a Nobel prize for the experiment. Good preparation for Electron Diffraction from a Crystal Lattice and subsequent Advanced Laboratory experiments. Emphasis is on determining the interdependence of experimental parameters (e.g., accelerating voltage, magnetic field, radius, charge) through the Lorentz Force Law. A good beginning lab. THERMODYNAMICS/STATISTICAL MECHANICS T1. Velocity and Gravitational Distributions An excellent introduction to the kinetic theory of gases. Uses an air table to investigate the statistical distribution of the velocity of particles. Compares the results with Statistical Mechanics velocity distribution functions. Also explores the effect of an external field (gravity) on the statistical distribution of particles. Emphasizes statistical modeling. Computer interfacing uses a video camera. -

Ballistic Pendulum March 2021 Lancaster/Basnet/Brown
Ballistic Pendulum March 2021 Lancaster/Basnet/Brown 1 Introduction This lab simulation explores the concept of conservation of energy by way of a ballistic pendulum and a projectile. 1.1 Tools This lab requires the use of the Google Chrome browser. Make certain it is installed on your computer. Navigate to http://physics.bu.edu/~duffy/HTML5/ballistic_pendulum.html to use the simulation. Intall the following Google Chrome extensions: Measure-it and Web Paint. They can be found at: https://chrome.google.com/webstore/detail/measure-it/jocbgkoackihphodedlefohapackjmna? hl=en https://chrome.google.com/webstore/detail/web-paint/emeokgokialpjadjaoeiplmnkjoaegng? hl=en. 1.2 Relevant Equations Kinetic energy: 1 K = mv2 (1) 2 Gravitational potential energy: U = mgh (2) Total energy: 1 E = K + U = mv2 + mgh (3) T otal 2 The velocity of the bullet-pendulum system immediately after the collision is: mb v = vb( ) (4) mb + M Where mb and vb are the mass and initial velocity of the bullet, and M is the mass of the ball hanging at the end of the pendulum. The kinetic energy of the bullet-pendulum system immediately after the collision is: 2 2 1 mb 2 2 1 mb vb K = (mb + M)( ) vb = (5) 2 mb + M 2 (mb + M) 2 Procedure Note: This simulation moves fast. For this reason, we'll take data using successive presses of the "step" button. Another note: For the purposes of this lab, assume that the initial gravitational potential of the pendulum is 0. 1 Ballistic Pendulum March 2021 Lancaster/Basnet/Brown 2.1 Part I: Initial Measurements 1. -

The Dawn of Fluid Dynamics a Discipline Between Science and Technology
Titelei Eckert 11.04.2007 14:04 Uhr Seite 3 Michael Eckert The Dawn of Fluid Dynamics A Discipline between Science and Technology WILEY-VCH Verlag GmbH & Co. KGaA Titelei Eckert 11.04.2007 14:04 Uhr Seite 1 Michael Eckert The Dawn of Fluid Dynamics A Discipline between Science and Technology Titelei Eckert 11.04.2007 14:04 Uhr Seite 2 Related Titles R. Ansorge Mathematical Models of Fluiddynamics Modelling, Theory, Basic Numerical Facts - An Introduction 187 pages with 30 figures 2003 Hardcover ISBN 3-527-40397-3 J. Renn (ed.) Albert Einstein - Chief Engineer of the Universe 100 Authors for Einstein. Essays approx. 480 pages 2005 Hardcover ISBN 3-527-40574-7 D. Brian Einstein - A Life 526 pages 1996 Softcover ISBN 0-471-19362-3 Titelei Eckert 11.04.2007 14:04 Uhr Seite 3 Michael Eckert The Dawn of Fluid Dynamics A Discipline between Science and Technology WILEY-VCH Verlag GmbH & Co. KGaA Titelei Eckert 11.04.2007 14:04 Uhr Seite 4 The author of this book All books published by Wiley-VCH are carefully produced. Nevertheless, authors, editors, and Dr. Michael Eckert publisher do not warrant the information Deutsches Museum München contained in these books, including this book, to email: [email protected] be free of errors. Readers are advised to keep in mind that statements, data, illustrations, proce- Cover illustration dural details or other items may inadvertently be “Wake downstream of a thin plate soaked in a inaccurate. water flow” by Henri Werlé, with kind permission from ONERA, http://www.onera.fr Library of Congress Card No.: applied for British Library Cataloging-in-Publication Data: A catalogue record for this book is available from the British Library. -

Elegant Solutions
Book Reviews 157 THE END OF SILENT RITES the top ten list, you might respond. The list includes, of course, the most famous Philip Ball: Elegant Solutions: Ten heroes from the history of chemistry Beautiful Experiments in Chemistry , and captures their most important The Royal Society of Chemistry, breakthrough experiments. By copying Cambridge, UK, 2005, vii+212 pp. or transferring to current research issues [ISBN 0-85404-674-7] the heroic deeds of the past, young scholars can accomplish excellence. In 2002 the American Chemical Society Now, if the key to doing important (ACS) asked its members to submit chemistry is learning from the history of proposals for the “ten most beautiful chemistry, why did the ACS encourage experiments in chemistry” ( C&EN , doing history of chemistry in the clum- Nov. 18, 2002, p. 5) and then proudly siest manner one can imagine – by col- published the result of the vote in its lecting and ranking decontextualized Chemical and Engineering News maga- historical ‘facts’ and anecdotes from the zine ( C&EN , Aug. 25, 2003, pp. 27-30). memory of its members who used to Democratic as the procedure is, it avoids have no training in the history of chem- asking critical questions: What is an ex- istry? Because the scholarly historiog- periment? What is beauty? What is raphy of chemistry does not matter chemistry? In fact, you need not be able here, you might respond. What matters to give an answer to these questions in is only what today’s chemists consider order to vote. We could even imagine to be important chemistry of the past, none of the voters being able to answer be that invented anecdotes or not. -

Ballistic Pendulum Is a Wonderful Way of Demonstrating Both the Constant Horizontal Velocity in Projectile Motion and the Conservation of Momentum
Conservation of Momentum Objective: To study the conservation of energy and momentum using projectile motion. Theory: The ballistic pendulum is a wonderful way of demonstrating both the constant horizontal velocity in projectile motion and the conservation of momentum. Because there is no acceleration in the horizontal direction, the horizontal component vx of the projectile’s velocity remains unchanged from its initial value throughout the motion. In a closed isolated system if no net external force acts on a system of particles, the total linear momentum of the system cannot change. There are two simple types of collisions, elastic and inelastic. If the total kinetic energy of the two systems is conserved then the collision is known as elastic. If the kinetic energy is not conserved then the collision is inelastic. Apparatus: PASCO Ballistic pendulum, meter stick, level, carbon paper, sheet of white paper, plumb bob, clamp. Procedure: Warning: Do not under any circumstance look into the barrel of the launcher. The people carrying out the experiment should wear safety googles at all times. Part 1: Projectile Motion – The goal is to determine the initial velocity vx of the ball 1. Make sure the pendulum is secured by the clamp and does not slide on the table. 2. Lift the pendulum arm until it is fully horizontal so that the ball can freely fire from the spring gun. 3. Set the apparatus near the edge of a table and level the apparatus. If the plump bob is fully vertical and overlapping with the 0 degree line it means it is properly leveled. -

Conservation of Linear Momentum: the Ballistic Pendulum
Conservation of Linear Momentum: the Ballistic Pendulum I. Discussion a. Determination of Velocity from Collision In the typical use made of a ballistic pendulum, a projectile, having a small mass, m, and a horizontal velocity, v, strikes and imbeds itself in a pendulum bob, having a large mass, M, and an initial horizontal velocity of zero. Immediately after the collision occurs the combined mass, M + m, of the bob and projectile has a small horizontal velocity, V. During this collision the conservation of linear momentum holds. If rotational effects are ignored, and if it is assumed that the time required for this event to take place is too short to allow the projectile to be substantially influenced by the earth's gravitational field, the motion remains horizontal, and mv = ( M + m ) V (1) After the collision has occurred, the combined mass, (M + m), rises along a circular arc to a vertical height h in the earth's gravitational field, as shown in Fig. l (c). During this portion of the motion, but not during the collision itself, the conservation of energy holds, if it is assumed that the effects of friction are negligible. Then, for motion along the arc, 1 2 ( M + m ) V = ( M + m) gh , or (2) 2 V = 2 gh (3) When V is eliminated using equations 1 and 3, the velocity of the projectile is v = M + m 2 gh (4) m b. Determination of Velocity from Range When a projectile, having an initial horizontal velocity, v, is fired from a height H above a plane, its horizontal range, R, is given by R = v t x o (5) and the height H is given by 1 2 H = gt (6) 2 where t = time during which projectile is in flight. -
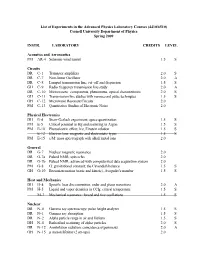
List of Experiments in the Advanced Physics Laboratory Courses (4410/6510) Cornell University Department of Physics Spring 2009
List of Experiments in the Advanced Physics Laboratory Courses (4410/6510) Cornell University Department of Physics Spring 2009 INSTR. LABORATORY CREDITS LEVEL Acoustics and Aeronautics PM AR-4 Subsonic wind tunnel 1.5 S Circuits DR C-1 Transistor amplifiers 2.0 S DR C-7 Non-linear Oscillator 2.0 A DR C-8 Lumped transmission line; cut-off and dispersion 1.5 S GH C-9 Radio frequency transmission line study 2.0 A DR C-10 Microwaves: components, phenomena, optical characteristics 2.0 S GH C-11 Transmission line studies with nanosecond pulse techniques 1.5 S GH C-12 Microwave Resonant Circuits 2.0 PM C-13 Quantitative Studies of Electronic Noise 2.0 Physical Electronics DH E-4 Stern-Gerlach experiment, space quantization 1.5 S PM E-5 Critical potential in Hg and scattering in Argon 1.5 S PM E-10 Photoelectric effect; h/e, Einstein relation 1.5 S E-12 Electron lens: magnetic and electrostatic types 1.5 S PM E-15 e/M: mass spectrograph with alkali metal ions 2.0 General DR G-7 Nuclear magnetic resonance 2.0 DR G-7a Pulsed NMR; spin echo 2.0 DR G-7b Pulsed NMR; advanced with computerized data acquisition system 2.0 PM G-8 G: gravitational constant; the Cavendish balance 1.5 S GH G-10 Brownian motion (static and kinetic), Avogadro's number 1.5 S Heat and Mechanics DH H-4 Specific heat discontinuities; order and phase transitions 2.0 A PM H-5 Liquid and vapor densities in CCl4; critical temperature 1.5 S M-3 Mechanical resonance: forced and free oscillations 1.5 S Nuclear DR N-0 Gamma ray spectroscopy: pulse height analyzer 1.5 S DR N-1 Gamma -

The Captive Lab Rat: Human Medical Experimentation in the Carceral State
Boston College Law Review Volume 61 Issue 1 Article 2 1-29-2020 The Captive Lab Rat: Human Medical Experimentation in the Carceral State Laura I. Appleman Willamette University, [email protected] Follow this and additional works at: https://lawdigitalcommons.bc.edu/bclr Part of the Bioethics and Medical Ethics Commons, Criminal Law Commons, Disability Law Commons, Health Law and Policy Commons, Juvenile Law Commons, Law and Economics Commons, Law and Society Commons, Legal History Commons, and the Medical Jurisprudence Commons Recommended Citation Laura I. Appleman, The Captive Lab Rat: Human Medical Experimentation in the Carceral State, 61 B.C.L. Rev. 1 (2020), https://lawdigitalcommons.bc.edu/bclr/vol61/iss1/2 This Article is brought to you for free and open access by the Law Journals at Digital Commons @ Boston College Law School. It has been accepted for inclusion in Boston College Law Review by an authorized editor of Digital Commons @ Boston College Law School. For more information, please contact [email protected]. THE CAPTIVE LAB RAT: HUMAN MEDICAL EXPERIMENTATION IN THE CARCERAL STATE LAURA I APPLEMAN INTRODUCTION ................................................................................................................................ 2 I. A HISTORY OF CAPTIVITY AND EXPERIMENTATION .................................................................... 4 A. Asylums and Institutions ........................................................................................................ 5 B. Orphanages, Foundling -

INTERMEDIATE LABORATORY - PHYX 3870-3880 Description of Experiments
INTERMEDIATE LABORATORY - PHYX 3870-3880 Description of Experiments MECHANICS M1. Kater's Pendulum A straightforward lab using a reversible physical pendulum to measure the local acceleration of gravity, g, to less than 0.01%. Emphasis is given to detailed error analysis and error propagation and to high precision measurements. Computer interfacing with photogates and motion sensors. A good beginning lab. M2. Coupled Pendulum Investigation of two pendulums coupled by a spring between them. Lab emphasizes comparison of measured results of the periods and damping to values derived from a theoretical model using differential equations. Completion of Analytical Mechanics is recommended. Computerized data collection with voltage probes and motion sensors and data reduction are used. M3. Cavendish Balance Determines the universal gravitational constant, G, using a torsion balance to measure the attraction between known masses. Emphasis is on fitting the data with a detailed model and extracting results by correlating the fitting parameters with the physical model. A good beginning lab. ELECTRICITY & MAGNETISM E1. Millikan's Oil Drop Experiment Demonstrates the quantized nature of the electric charge and measures a value of the fundamental constant e. This classic experiment led to the first Nobel prize for an American physicist. Emphasizes experimental design and statistical analysis methods using large data sets. E2. Thompson's e/m Experiment Measures the ratio of the charge to mass of an electron (e/m) by investigating the trajectory of electrons in electric and magnetic fields. The British physicist J. J. Thompson received a Nobel prize for the experiment. Good preparation for Electron Diffraction from a Crystal Lattice and subsequent Advanced Laboratory experiments. -

Josiah Parsons Cooke
MEMOIR JOSIAH PARSONS COOKE. Q97 i CHARLES L. JACKSON. PREPARED FOR THE NATIONAL ACADEMY. (26) 175 BIOGRAPHICAL MEMOIR OF JOSIAH PARSONS COOKE. JOSIAH PARSONS COOKE, the son of Josiah Parsons Cooke, a successful lawyer, and Mary (Pratt) Cooke, was born in Boston October 12,1827. He was educated at the Boston Latin School and Harvard College, from which he graduated in 1848, and after a year of travel in Europe was appointed tutor in math- ematics in Harvard College in 1849. In 1850 he became Erving Professor of Chemistry and Mineralogy, a position which he held for the remainder of his life. Cooke's equipment for the duties of his new place was almost entirely the result of his own exertions. A course of lectures by the elder Silliman first aroused his enthusiasm for chemistry and led him in early boyhood to fit up a laboratory in his father's house, where he attacked the science by experiment with such good results that even when he came to college he had a work- ing knowledge of the subject. At Cambridge he continued these studies essentially alone, as the chemical teaching of the college during his four 3'ears of residence was confined to five or six rather disjointed and fragmentary lectures. Immediately after appointment to his professorship he supplemented these meager preparations by obtaining a leave of absence for eight months, which were spent in Europe buying apparatus and material and attending lectures by Regnault and Dumas. These formed the only instruction in chemistry he had received which could even claim to be systematic; yet with this slender outfit, aided by barely a year and a half of experience as a teacher, in 1851, at the age of 24, he found himself confronted with problems which would have taxed the abilities of an old, experienced, and fully educated professor.