Primary T Cell Immunodeficiencies Associated with Disturbed Proximal
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Overexpression of Syk Tyrosine Kinase in Peripheral T-Cell Lymphomas
Leukemia (2008) 22, 1139–1143 & 2008 Nature Publishing Group All rights reserved 0887-6924/08 $30.00 www.nature.com/leu ORIGINAL ARTICLE Overexpression of Syk tyrosine kinase in peripheral T-cell lymphomas AL Feldman1, DX Sun1, ME Law1, AJ Novak2, AD Attygalle3, EC Thorland1, SR Fink1, JA Vrana1, BL Caron1, WG Morice1, ED Remstein1, KL Grogg1, PJ Kurtin1, WR Macon1 and A Dogan1 1Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN, USA; 2Department of Hematology, Mayo Clinic, Rochester, MN, USA and 3Department of Histopathology, Royal Marsden Hospital, London, UK Peripheral T-cell lymphomas (PTCLs) are fatal in the majority of Materials and methods patients and novel treatments, such as protein tyrosine kinase (PTK) inhibition, are needed. The recent finding of SYK/ITK translocations in rare PTCLs led us to examine the expression Cases of Syk PTK in 141 PTCLs. Syk was positive by immuno- We studied specimens from 141 patients with PTCL diagnosed 15 histochemistry (IHC) in 133 PTCLs (94%), whereas normal by WHO criteria. There were 86 men and 55 women of a T cells were negative. Western blot on frozen tissue (n ¼ 6) mean age of 59 years (range, 5–88 years). The study was and flow cytometry on cell suspensions (n ¼ 4) correlated with approved by the Institutional Review Board and the Biospeci- IHC results in paraffin. Additionally, western blot demonstrated mens Committee of Mayo Clinic. All patients provided informed that Syk-positive PTCLs show tyrosine (525/526) phosphory- lation, known to be required for Syk activation. Fluorescence consent for the use of their tissues for research purposes. -

CD81 Antibody (C-Term) Purified Rabbit Polyclonal Antibody (Pab) Catalog # AP6631B
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 CD81 Antibody (C-term) Purified Rabbit Polyclonal Antibody (Pab) Catalog # AP6631B Specification CD81 Antibody (C-term) - Product Information Application WB, IHC-P, FC,E Primary Accession P60033 Reactivity Human, Mouse Host Rabbit Clonality Polyclonal Isotype Rabbit Ig Antigen Region 176-203 CD81 Antibody (C-term) - Additional Information Gene ID 975 Other Names CD81 antigen, 26 kDa cell surface protein TAPA-1, Target of the antiproliferative antibody 1, Tetraspanin-28, Tspan-28, Western blot analysis of CD81 Antibody CD81, CD81, TAPA1, TSPAN28 (C-term) (Cat. #AP6631b) in mouse kidney(lane 1) and cerebellum(lane 2) tissue Target/Specificity lysates (35ug/lane). CD81 (arrow) was This CD81 antibody is generated from detected using the purified Pab. rabbits immunized with a KLH conjugated synthetic peptide between 176-203 amino acids from the C-terminal region of human CD81. Dilution WB~~1:1000 IHC-P~~1:10~50 FC~~1:10~50 Format Purified polyclonal antibody supplied in PBS with 0.09% (W/V) sodium azide. This antibody is prepared by Saturated Ammonium Sulfate (SAS) precipitation followed by dialysis against PBS. Storage Maintain refrigerated at 2-8°C for up to 2 Formalin-fixed and paraffin-embedded weeks. For long term storage store at -20°C in small aliquots to prevent freeze-thaw human brain tissue with CD81 Antibody cycles. (C-term), which was peroxidase-conjugated to the secondary antibody, followed by DAB Precautions staining. This data demonstrates the use of CD81 Antibody (C-term) is for research use this antibody for immunohistochemistry; Page 1/4 10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 only and not for use in diagnostic or clinical relevance has not been evaluated. -

Exome Sequencing Identifies Mutations in the Gene TTC7A In
Developmental defects J Med Genet: first published as 10.1136/jmedgenet-2012-101483 on 19 February 2013. Downloaded from ORIGINAL ARTICLE Exome sequencing identifies mutations in the gene TTC7A in French-Canadian cases with hereditary multiple intestinal atresia Mark E Samuels,1 Jacek Majewski,2 Najmeh Alirezaie,2 Isabel Fernandez,1,3 Ferran Casals,1 Natalie Patey,1,4 Hélène Decaluwe,1,5 Isabelle Gosselin,6 Elie Haddad,1,3,5 Alan Hodgkinson,1 Youssef Idaghdour,1 Valerie Marchand,1,5 1,5 6,7 6 6 Open Access Jacques L Michaud, Marc-André Rodrigue, Sylvie Desjardins, Stéphane Dubois, Scan to access more 1,3 1,5 6,7 8 free content Francoise Le Deist, Philip Awadalla, Vincent Raymond, Bruno Maranda ▸ Additional material is ABSTRACT attempted, outcomes are poor and the condition is published online only. To view Background Congenital multiple intestinal atresia usual fatal within the first month of life. To date, please visit the journal online (http://dx.doi.org/10.1136/ (MIA) is a severe, fatal neonatal disorder, involving the no primary aetiology has been proved for the con- jmedgenet-2012-101483). occurrence of obstructions in the small and large dition. Importantly, in some cases MIA is also asso- 1 intestines ultimately leading to organ failure. Surgical ciated with either mild or severe combined Centre de Recherche du CHU fi 2–5 Ste-Justine, University of interventions are palliative but do not provide long-term immunode ciency (SCID), raising the possibility Montreal, Montreal, Quebec, survival. Severe immunodeficiency may be associated that an abnormal immune response might be the Canada with the phenotype. -

ITK Inhibitors for the Treatment of T-Cell Lymphoproliferative Disorders John C
ITK Inhibitors for the Treatment of T-Cell Lymphoproliferative Disorders John C. Reneau, MD, PhD1, Steven R. Hwang, MD2, Carlos A. Murga-Zamalloa, MD1, Joseph J. Buggy, PhD3, James W. Janc, PhD3 and Ryan A. Wilcox, MD, PhD1 1University of Michigan, Ann Arbor, MI; 2Mayo Clinic, Rochester, MN; 3Corvus Pharmaceuticals, Inc., Burlingame, CA Introduction Results and Methods Conclusions • T-cell lymphomas (TCL) comprise a rare, aggressive, and Table 1: CPI-818 specifically inhibits ITK Figure 2: CPI-818 has minimal effect on normal T cells • CPI-818 is a potent ITK specific inhibitor, while CPI-893 inhibits both heterogeneous subtype of non-Hodgkin lymphoma B IC50 (nM) A ITK and RLK • Outcomes for patients with TCL remain poor and novel therapies are ITK RLK • Normal T cells express both ITK and RLK which can compensate for needed CPI-818 (ITKi) 2.3 260 inhibition of ITK function CPI-893 (ITK/RLKi) 0.36 0.4 • Engagement of the T-cell receptor (TCR) in malignant T-cells leads to • Malignant T cells almost exclusively express ITK, or express RLK at Kinome screening was performed for CPI-818 (ITKi) and CPI-893 (ITK/RLKi). CPI-818 (ITKi) had high interleukin-2-inudcible T-cell kinase (ITK) dependent activation of NF- specificity for ITK over RLK (IC50 2.3 nM and 260 nM, respectively). In contrast CPI-893 (ITK/RLKi) had a very low levels high affinity for both ITK and RLK (IC50 0.36 nM and 0.4 nM, respectively) 1 κB and GATA3, and promotes chemotherapy resistance . (A) Peripheral blood T cells from healthy donors were isolated by negative selection. -
Primepcr™Assay Validation Report
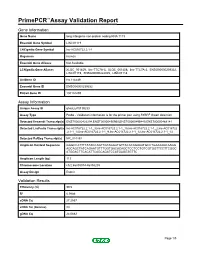
PrimePCR™Assay Validation Report Gene Information Gene Name long intergenic non-protein coding RNA 1119 Ensembl Gene Symbol LINC01119 LNCipedia Gene Symbol lnc-AC016722.2.1-1 Organism Human Ensembl Gene Aliases Not Available LCNipedia Gene Aliases XLOC_001459, linc-TTC7A-3, XLOC_001458, linc-TTC7A-2, ENSG00000239332, LINC01119, ENSG00000222005, LINC01118 UniGene ID Hs.114449 Ensembl Gene ID ENSG00000239332 Entrez Gene ID 100134259 Assay Information Unique Assay ID qhsaLEP0139233 Assay Type Probe - Validation information is for the primer pair using SYBR® Green detection Detected Ensembl Transcript(s) ENST00000422294,ENST00000490950,ENST00000495449,ENST00000468141 Detected LncPedia Transcript(s) lnc-AC016722.2.1-1_3,lnc-AC016722.2.1-1_15,lnc-AC016722.2.1-1_2,lnc-AC016722 .2.1-1_14,lnc-AC016722.2.1-1_9,lnc-AC016722.2.1-1_12,lnc-AC016722.2.1-1_13 Detected RefSeq Transcript(s) NR_024452 Amplicon Context Sequence GGGCCATTTTATACCAGTTGTAGGATGTTACACAGAAATGCCTGAAAAGCAAGG AGCAGCTATCAGAATGTTTGGTGACACAGCTCCTCCTGTCGTGGTTCCTTCGGC ATGGACTTCACATTCAGCAGATCCATGAGGTGTTC Amplicon Length (bp) 113 Chromosome Location chr2:46855974-46858205 Assay Design Exonic Validation Results Efficiency (%) 99.5 R2 0.9986 cDNA Cq 27.2537 cDNA Tm (Celsius) 83 gDNA Cq 24.0682 Page 1/5 PrimePCR™Assay Validation Report Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 2/5 PrimePCR™Assay Validation Report LINC01119, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal reference RNA Melt Peak -

Review Tec Kinases: a Family with Multiple Roles in Immunity
Immunity, Vol. 12, 373±382, April, 2000, Copyright 2000 by Cell Press Tec Kinases: A Family Review with Multiple Roles in Immunity Wen-Chin Yang,*³§ Yves Collette,*³ inositol phosphates, but they are thought to be relevant Jacques A. NuneÁ s,*³ and Daniel Olive*² for binding of PtdIns lipids to the same sites. In most *INSERM U119 cases, PH domains bind preferentially to PtdIns (4,5)P2 Universite de la Me diterrane e and inositol (1,4,5) P3 (Ins (1,4,5) P3). However, the Btk 13009 Marseille PH domain binds PtdIns (3,4,5)P3 and Ins (1, 3, 4, 5)P4 France the tightest. PtdIns (3, 4, 5)P3, one of the products of the action of PI3K, is thought to act as a second messen- ger to recruit regulatory proteins to the plasma mem- brane via their PH domains (see below). Many of the Antigen receptors on T, B, and mast cells are multimo- mutations in Btk that lead to XLA are point mutations lecular complexes that are activated by interactions with that cluster at one end of the PH domain and could be external signals. These signals are then transmitted to predicted to impair binding to Ins (3,4,5)P (for review regulate gene expression and posttranscriptional modi- 3 see Satterthwaite et al., 1998a) (Figure 1b). Similarly, fications. Nonreceptor tyrosine kinases (NRTK) are key CBA/N xid mice carry an R28C mutation in the Btk PH players that relay and integrate these signals. NRTK are domain. The recent structure of the PH domain from divided into distinct families defined by a prototypic Btk complexed with Ins (1,3,4,5)P4 provides an explana- member: Src, Tec, Syk, Csk, Fes, Abl, Jak, Fak, Ack, tion for several mutations associated with XLA: mis- Brk, and Srm (Bolen and Brugge, 1997). -

CD226 T Cells Expressing the Receptors TIGIT and Divergent Phenotypes of Human Regulatory
The Journal of Immunology Divergent Phenotypes of Human Regulatory T Cells Expressing the Receptors TIGIT and CD226 Christopher A. Fuhrman,*,1 Wen-I Yeh,*,1 Howard R. Seay,* Priya Saikumar Lakshmi,* Gaurav Chopra,† Lin Zhang,* Daniel J. Perry,* Stephanie A. McClymont,† Mahesh Yadav,† Maria-Cecilia Lopez,‡ Henry V. Baker,‡ Ying Zhang,x Yizheng Li,{ Maryann Whitley,{ David von Schack,x Mark A. Atkinson,* Jeffrey A. Bluestone,‡ and Todd M. Brusko* Regulatory T cells (Tregs) play a central role in counteracting inflammation and autoimmunity. A more complete understanding of cellular heterogeneity and the potential for lineage plasticity in human Treg subsets may identify markers of disease pathogenesis and facilitate the development of optimized cellular therapeutics. To better elucidate human Treg subsets, we conducted direct transcriptional profiling of CD4+FOXP3+Helios+ thymic-derived Tregs and CD4+FOXP3+Helios2 T cells, followed by comparison with CD4+FOXP32Helios2 T conventional cells. These analyses revealed that the coinhibitory receptor T cell Ig and ITIM domain (TIGIT) was highly expressed on thymic-derived Tregs. TIGIT and the costimulatory factor CD226 bind the common ligand CD155. Thus, we analyzed the cellular distribution and suppressive activity of isolated subsets of CD4+CD25+CD127lo/2 T cells expressing CD226 and/or TIGIT. We observed TIGIT is highly expressed and upregulated on Tregs after activation and in vitro expansion, and is associated with lineage stability and suppressive capacity. Conversely, the CD226+TIGIT2 population was associated with reduced Treg purity and suppressive capacity after expansion, along with a marked increase in IL-10 and effector cytokine production. These studies provide additional markers to delineate functionally distinct Treg subsets that may help direct cellular therapies and provide important phenotypic markers for assessing the role of Tregs in health and disease. -

The Capacity of Long-Term in Vitro Proliferation of Acute Myeloid
The Capacity of Long-Term in Vitro Proliferation of Acute Myeloid Leukemia Cells Supported Only by Exogenous Cytokines Is Associated with a Patient Subset with Adverse Outcome Annette K. Brenner, Elise Aasebø, Maria Hernandez-Valladares, Frode Selheim, Frode Berven, Ida-Sofie Grønningsæter, Sushma Bartaula-Brevik and Øystein Bruserud Supplementary Material S2 of S31 Table S1. Detailed information about the 68 AML patients included in the study. # of blasts Viability Proliferation Cytokine Viable cells Change in ID Gender Age Etiology FAB Cytogenetics Mutations CD34 Colonies (109/L) (%) 48 h (cpm) secretion (106) 5 weeks phenotype 1 M 42 de novo 241 M2 normal Flt3 pos 31.0 3848 low 0.24 7 yes 2 M 82 MF 12.4 M2 t(9;22) wt pos 81.6 74,686 low 1.43 969 yes 3 F 49 CML/relapse 149 M2 complex n.d. pos 26.2 3472 low 0.08 n.d. no 4 M 33 de novo 62.0 M2 normal wt pos 67.5 6206 low 0.08 6.5 no 5 M 71 relapse 91.0 M4 normal NPM1 pos 63.5 21,331 low 0.17 n.d. yes 6 M 83 de novo 109 M1 n.d. wt pos 19.1 8764 low 1.65 693 no 7 F 77 MDS 26.4 M1 normal wt pos 89.4 53,799 high 3.43 2746 no 8 M 46 de novo 26.9 M1 normal NPM1 n.d. n.d. 3472 low 1.56 n.d. no 9 M 68 MF 50.8 M4 normal D835 pos 69.4 1640 low 0.08 n.d. -

TTC7A Mutations Disrupt Intestinal Epithelial Apicobasal Polarity
TTC7A mutations disrupt intestinal epithelial apicobasal polarity Amélie E. Bigorgne, … , Hans Clevers, Geneviève de Saint Basile J Clin Invest. 2014;124(1):328-337. https://doi.org/10.1172/JCI71471. Research Article Gastroenterology Multiple intestinal atresia (MIA) is a rare cause of bowel obstruction that is sometimes associated with a combined immunodeficiency (CID), leading to increased susceptibility to infections. The factors underlying this rare disease are poorly understood. We characterized the immunological and intestinal features of 6 unrelated MIA-CID patients. All patients displayed a profound, generalized lymphocytopenia, with few lymphocytes present in the lymph nodes. The thymus was hypoplastic and exhibited an abnormal distribution of epithelial cells. Patients also had profound disruption of the epithelial barrier along the entire gastrointestinal tract. Using linkage analysis and whole-exome sequencing, we identified 10 mutations in tetratricopeptide repeat domain–7A (TTC7A), all of which potentially abrogate TTC7A expression. Intestinal organoid cultures from patient biopsies displayed an inversion of apicobasal polarity of the epithelial cells that was normalized by pharmacological inhibition of Rho kinase. Our data indicate that TTC7A deficiency results in increased Rho kinase activity, which disrupts polarity, growth, and differentiation of intestinal epithelial cells, and which impairs immune cell homeostasis, thereby promoting MIA-CID development. Find the latest version: https://jci.me/71471/pdf Research article TTC7A mutations disrupt intestinal epithelial apicobasal polarity Amélie E. Bigorgne,1,2,3 Henner F. Farin,4 Roxane Lemoine,1,2,3 Nizar Mahlaoui,2,3 Nathalie Lambert,5 Marine Gil,5 Ansgar Schulz,6 Pierre Philippet,7 Patrick Schlesser,7 Tore G. Abrahamsen,8 Knut Oymar,9 E. -

CD Markers Are Routinely Used for the Immunophenotyping of Cells
ptglab.com 1 CD MARKER ANTIBODIES www.ptglab.com Introduction The cluster of differentiation (abbreviated as CD) is a protocol used for the identification and investigation of cell surface molecules. So-called CD markers are routinely used for the immunophenotyping of cells. Despite this use, they are not limited to roles in the immune system and perform a variety of roles in cell differentiation, adhesion, migration, blood clotting, gamete fertilization, amino acid transport and apoptosis, among many others. As such, Proteintech’s mini catalog featuring its antibodies targeting CD markers is applicable to a wide range of research disciplines. PRODUCT FOCUS PECAM1 Platelet endothelial cell adhesion of blood vessels – making up a large portion molecule-1 (PECAM1), also known as cluster of its intracellular junctions. PECAM-1 is also CD Number of differentiation 31 (CD31), is a member of present on the surface of hematopoietic the immunoglobulin gene superfamily of cell cells and immune cells including platelets, CD31 adhesion molecules. It is highly expressed monocytes, neutrophils, natural killer cells, on the surface of the endothelium – the thin megakaryocytes and some types of T-cell. Catalog Number layer of endothelial cells lining the interior 11256-1-AP Type Rabbit Polyclonal Applications ELISA, FC, IF, IHC, IP, WB 16 Publications Immunohistochemical of paraffin-embedded Figure 1: Immunofluorescence staining human hepatocirrhosis using PECAM1, CD31 of PECAM1 (11256-1-AP), Alexa 488 goat antibody (11265-1-AP) at a dilution of 1:50 anti-rabbit (green), and smooth muscle KD/KO Validated (40x objective). alpha-actin (red), courtesy of Nicola Smart. PECAM1: Customer Testimonial Nicola Smart, a cardiovascular researcher “As you can see [the immunostaining] is and a group leader at the University of extremely clean and specific [and] displays Oxford, has said of the PECAM1 antibody strong intercellular junction expression, (11265-1-AP) that it “worked beautifully as expected for a cell adhesion molecule.” on every occasion I’ve tried it.” Proteintech thanks Dr. -

Itk Promotes the Integration of TCR and CD28 Costimulation, Through Its
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.11.293316; this version posted September 11, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Hallumi et al. 1 Article 2 Itk promotes the integration of TCR and CD28 3 costimulation, through its direct substrates, SLP-76 4 and Gads 5 Enas Hallumi1, Rose Shalah1, Wan-Lin Lo2, Jasmin Corso3, Ilana Oz1, Dvora Beach1, Samuel 6 Wittman1, Amy Isenberg1, Meirav Sela1, Henning Urlaub3,4, Arthur Weiss2,5 and Deborah 7 Yablonski1* 8 1 Department of Immunology, Ruth and Bruce Rappaport Faculty of Medicine, Technion—Israel Institute of 9 Technology, Haifa 3525433, Israel 10 2 Division of Rheumatology, Rosalind Russell and Ephraim P. Engleman Arthritis Research Center, 11 Department of Medicine, University of California, San Francisco, San Francisco, CA, USA 12 3 Bioanalytical Mass Spectrometry Group, Max Planck Institute for Biophysical Chemistry, Am Fassberg 11, 13 37077 Göttingen, Germany 14 4 Bioanalytics, Institute for Clinical Chemistry, University Medical Center Göttingen, Robert Koch Strasse 40, 15 37075 Göttingen, Germany 16 5 Howard Hughes Medical Institute, University of California, San Francisco, San Francisco, CA, USA 17 * Correspondence: [email protected] 18 Abstract: The costimulatory receptor, CD28, synergizes with the T cell antigen receptor (TCR) to 19 promote IL-2 production, cell survival and proliferation. Despite their profound synergy, the 20 obligatory interdependence of the signaling pathways initiated by these two receptors is not well 21 understood. Upon TCR stimulation, Gads, a Grb2-family adaptor, bridges the interaction of two 22 additional adaptors, LAT and SLP-76, to form a TCR-induced effector signaling complex. -
HCC and Cancer Mutated Genes Summarized in the Literature Gene Symbol Gene Name References*
HCC and cancer mutated genes summarized in the literature Gene symbol Gene name References* A2M Alpha-2-macroglobulin (4) ABL1 c-abl oncogene 1, receptor tyrosine kinase (4,5,22) ACBD7 Acyl-Coenzyme A binding domain containing 7 (23) ACTL6A Actin-like 6A (4,5) ACTL6B Actin-like 6B (4) ACVR1B Activin A receptor, type IB (21,22) ACVR2A Activin A receptor, type IIA (4,21) ADAM10 ADAM metallopeptidase domain 10 (5) ADAMTS9 ADAM metallopeptidase with thrombospondin type 1 motif, 9 (4) ADCY2 Adenylate cyclase 2 (brain) (26) AJUBA Ajuba LIM protein (21) AKAP9 A kinase (PRKA) anchor protein (yotiao) 9 (4) Akt AKT serine/threonine kinase (28) AKT1 v-akt murine thymoma viral oncogene homolog 1 (5,21,22) AKT2 v-akt murine thymoma viral oncogene homolog 2 (4) ALB Albumin (4) ALK Anaplastic lymphoma receptor tyrosine kinase (22) AMPH Amphiphysin (24) ANK3 Ankyrin 3, node of Ranvier (ankyrin G) (4) ANKRD12 Ankyrin repeat domain 12 (4) ANO1 Anoctamin 1, calcium activated chloride channel (4) APC Adenomatous polyposis coli (4,5,21,22,25,28) APOB Apolipoprotein B [including Ag(x) antigen] (4) AR Androgen receptor (5,21-23) ARAP1 ArfGAP with RhoGAP domain, ankyrin repeat and PH domain 1 (4) ARHGAP35 Rho GTPase activating protein 35 (21) ARID1A AT rich interactive domain 1A (SWI-like) (4,5,21,22,24,25,27,28) ARID1B AT rich interactive domain 1B (SWI1-like) (4,5,22) ARID2 AT rich interactive domain 2 (ARID, RFX-like) (4,5,22,24,25,27,28) ARID4A AT rich interactive domain 4A (RBP1-like) (28) ARID5B AT rich interactive domain 5B (MRF1-like) (21) ASPM Asp (abnormal