Understanding Chemotherapy Information for Patients and Their Loved Ones
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cancer Drug Pharmacology Table
CANCER DRUG PHARMACOLOGY TABLE Cytotoxic Chemotherapy Drugs are classified according to the BC Cancer Drug Manual Monographs, unless otherwise specified (see asterisks). Subclassifications are in brackets where applicable. Alkylating Agents have reactive groups (usually alkyl) that attach to Antimetabolites are structural analogues of naturally occurring molecules DNA or RNA, leading to interruption in synthesis of DNA, RNA, or required for DNA and RNA synthesis. When substituted for the natural body proteins. substances, they disrupt DNA and RNA synthesis. bendamustine (nitrogen mustard) azacitidine (pyrimidine analogue) busulfan (alkyl sulfonate) capecitabine (pyrimidine analogue) carboplatin (platinum) cladribine (adenosine analogue) carmustine (nitrosurea) cytarabine (pyrimidine analogue) chlorambucil (nitrogen mustard) fludarabine (purine analogue) cisplatin (platinum) fluorouracil (pyrimidine analogue) cyclophosphamide (nitrogen mustard) gemcitabine (pyrimidine analogue) dacarbazine (triazine) mercaptopurine (purine analogue) estramustine (nitrogen mustard with 17-beta-estradiol) methotrexate (folate analogue) hydroxyurea pralatrexate (folate analogue) ifosfamide (nitrogen mustard) pemetrexed (folate analogue) lomustine (nitrosurea) pentostatin (purine analogue) mechlorethamine (nitrogen mustard) raltitrexed (folate analogue) melphalan (nitrogen mustard) thioguanine (purine analogue) oxaliplatin (platinum) trifluridine-tipiracil (pyrimidine analogue/thymidine phosphorylase procarbazine (triazine) inhibitor) -

ASTRO Bone Metastases Guideline-Full Version
1 Palliative Radiotherapy for Bone Metastases: An ASTRO Evidence-Based Guideline Stephen T. Lutz, M.D.,* Lawrence B. Berk, M.D., Ph.D.,† Eric L. Chang, M.D.,‡ Edward Chow, M.B.B.S.,§ Carol A. Hahn, M.D.,║ Peter J. Hoskin, M.D.,¶ David D. Howell, M.D.,# Andre A. Konski, M.D.,** Lisa A. Kachnic, M.D.,†† Simon S. Lo, M.B. ChB,§§ Arjun Sahgal, M.D.,║║ Larry N. Silverman, M.D.,¶¶ Charles von Gunten, M.D., Ph.D., FACP,## Ehud Mendel, M.D., FACS,*** Andrew D. Vassil, M.D.,††† Deborah Watkins Bruner, R.N., Ph.D.,‡‡‡ and William F. Hartsell, M.D.§§§ * Department of Radiation Oncology, Blanchard Valley Regional Cancer Center, Findlay, Ohio; † Department of Radiation Oncology, Moffitt Cancer Center, Tampa, Florida; ‡ Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, Texas; § Department of Radiation Oncology, Sunnybrook Odette Cancer Center, University of Toronto, Toronto, Ontario, Canada; ║ Department of Radiation Oncology, Duke University, Durham, North Carolina; ¶ Mount Vernon Centre for Cancer Treatment, Middlesex, UK; # Department of Radiation Oncology, University of Michigan, Mt. Pleasant, Michigan; ** Department of Radiation Oncology, Wayne State University, Detroit, Michigan; †† Department of Radiation Oncology, Boston Medical Center, Boston, Massachusetts; §§ Department of Radiation Oncology, Ohio State University, Columbus, Ohio; ║║ Department of Radiation Oncology, Sunnybrook Odette Cancer Center and the Princess Margaret Hospital, University of Toronto, Toronto, Ontario, Canada; ¶¶ 21st Century Oncology, Sarasota, Florida; ## The Institute for Palliative Medicine, San Diego Hospice, San Diego, California; *** Neurological Surgery, Ohio State University, Columbus, Ohio; ††† Department of Radiation Oncology, The Cleveland Clinic 2 Foundation, Cleveland, Ohio; ‡‡‡ School of Nursing, University of Pennsylvania, Philadelphia, Pennsylvania; §§§ Department of Radiation Oncology, Good Samaritan Cancer Center, Downers Grove, Illinois Reprint requests to: Stephen Lutz, M.D., 15990 Medical Drive South, Findlay, OH 45840. -

In Vivo Characterization of Combination Antitumor Chemotherapy with Calcium Channel Blockers and Ci5-Diamminedichloroplatinum(II)1
(CANCER RESEARCH 49, 2844-2850. June 1, 1989] In Vivo Characterization of Combination Antitumor Chemotherapy with Calcium Channel Blockers and ci5-Diamminedichloroplatinum(II)1 James M. Onoda,2 Kevin K. Nelson, John D. Taylor, and Kenneth V. Honn Departments of Radiation Oncology [J. M. O., K. K. N., J. D. T., K. V. H.], Biological Sciences [J. D. T.], and Chemistry ¡K.V. H.], Wayne State University, Detroit, Michigan 48202; and the Cershenson Radiation Oncology Center [J. M. O., K. K. N., J. D. T., K. V. H.], Harper/Grace Hospitals, Detroit, Michigan 4820I ABSTRACT dulin antagonists to enhance the antitumor actions of the more We have examined nifedipine, a dihydropyridine class calcium channel commonly prescribed organic or natural product chemothera blocker, for ability to overcome m-diamminedichloroplatinum(II) (cis- peutic agents (3, 4). The ability of verapamil to reverse multi- platin) resistance in a murine tumor line variant, B16a-Pt, which we drug resistance or pleiotropic drug resistance correlates with developed for resistance to cisplatin. Nifedipine significantly enhanced the expression of a M, 170,000 glycoprotein in drug-resistant the antitumor actions of cisplatin against primary subcutaneous B16a-Pt tumor cell plasma membranes (5, 6). This glycoprotein is now tumors and their spontaneous pulmonary métastases.We have charac commonly referred to as the P-glycoprotein (7, 8) and is re terized, in vivo, the pharmacokinetics and dose-response interactions sponsible for the active efflux of many organic/natural product between nifedipine and cisplatin. We now report our studies designed to cytotoxic chemotherapeutic agents (9-11). The current hypoth compare, in vivo, the efficacy of nifedipine and other calcium active esis suggests that verapamil interacts with the P-glycoprotein compounds including: (a) structurally similar calcium channel blockers to block drug efflux (12, 13); and that its actions are independ (nimodipine, nicardipine) from the dihydropyridine class, (b) structurally ent of the classical slow-inward Ca2+ channel (14, 15). -

Cancer Treatment and Survivorship Facts & Figures 2019-2021
Cancer Treatment & Survivorship Facts & Figures 2019-2021 Estimated Numbers of Cancer Survivors by State as of January 1, 2019 WA 386,540 NH MT VT 84,080 ME ND 95,540 59,970 38,430 34,360 OR MN 213,620 300,980 MA ID 434,230 77,860 SD WI NY 42,810 313,370 1,105,550 WY MI 33,310 RI 570,760 67,900 IA PA NE CT 243,410 NV 185,720 771,120 108,500 OH 132,950 NJ 543,190 UT IL IN 581,350 115,840 651,810 296,940 DE 55,460 CA CO WV 225,470 1,888,480 KS 117,070 VA MO MD 275,420 151,950 408,060 300,200 KY 254,780 DC 18,750 NC TN 470,120 AZ OK 326,530 NM 207,260 AR 392,530 111,620 SC 143,320 280,890 GA AL MS 446,900 135,260 244,320 TX 1,140,170 LA 232,100 AK 36,550 FL 1,482,090 US 16,920,370 HI 84,960 States estimates do not sum to US total due to rounding. Source: Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute. Contents Introduction 1 Long-term Survivorship 24 Who Are Cancer Survivors? 1 Quality of Life 24 How Many People Have a History of Cancer? 2 Financial Hardship among Cancer Survivors 26 Cancer Treatment and Common Side Effects 4 Regaining and Improving Health through Healthy Behaviors 26 Cancer Survival and Access to Care 5 Concerns of Caregivers and Families 28 Selected Cancers 6 The Future of Cancer Survivorship in Breast (Female) 6 the United States 28 Cancers in Children and Adolescents 9 The American Cancer Society 30 Colon and Rectum 10 How the American Cancer Society Saves Lives 30 Leukemia and Lymphoma 12 Research 34 Lung and Bronchus 15 Advocacy 34 Melanoma of the Skin 16 Prostate 16 Sources of Statistics 36 Testis 17 References 37 Thyroid 19 Acknowledgments 45 Urinary Bladder 19 Uterine Corpus 21 Navigating the Cancer Experience: Treatment and Supportive Care 22 Making Decisions about Cancer Care 22 Cancer Rehabilitation 22 Psychosocial Care 23 Palliative Care 23 Transitioning to Long-term Survivorship 23 This publication attempts to summarize current scientific information about Global Headquarters: American Cancer Society Inc. -

The Immunosuppressive Effects of Long-Term Combination Chemotherapy in Children with Acute Leukemia Inremission1
[CANCER RESEARCH 31, 420-426, April 1971] The Immunosuppressive Effects of Long-Term Combination Chemotherapy in Children with Acute Leukemia in Remission1 Luis Borella and Robert G. Webster Laboratories of Virology and Immunology, St. Jude Children 'sResearch Hospital, Memphis, Tennessee 38101 SUMMARY effects of prolonged maintained combination chemotherapy upon immunocompetence have not been investigated. The immunosuppressive effects of maintenance Knowledge of the immunosuppressive effects of long-term combination chemotherapy given for periods ranging from 8 combination chemotherapy is now urgently needed because of to 28 months to 20 children with acute lymphocytic leukemia the increasing number of prolonged remissions and potential in remission were investigated. There was depression of both 5-year cures among children with acute lymphocytic leukemia the primary antibody production to the hemagglutinin antigen receiving this form of treatment (19). of the Hong Kong influenza virus and the anamnestic response This study was aimed at determining the to the neuraminidase of the same virus. The primary response immunosuppressive effects of long-term combination (hemagglutination inhibition) was affected to a greater extent chemotherapy in children with acute lymphocytic leukemia. than the secondary (neuraminidase inhibition) response. A Several unique features of this investigation were as follows. preferential depression of 2-mercaptoethanol-resistant (a) All patients were in remission and received the same hemagglutination inhibition antibodies (IgG) was also combination chemotherapy continuously for periods ranging observed. One-fourth of all acute lymphocytic leukemia from 8 to 28 months, (b) Patients and controls were patients had low serum IgG levels. In vitro transformation of immunized with the Hong Kong influenza virus vaccine prior lymphocytes was a poor index of immunocompetence. -

Predictors of Survival in Acute Myeloid Leukemia by Treatment Modality
ANTICANCER RESEARCH 36: 1719-1728 (2016) Predictors of Survival in Acute Myeloid Leukemia by Treatment Modality SAMIP MASTER1, RICHARD MANSOUR1, SRINIVAS S. DEVARAKONDA1, ZHENZHEN SHI2, GLENN MILLS1 and RUNHUA SHI1 1Department of Medicine & Feist-Weiller Cancer Center, Louisiana State University Health Shreveport, Shreveport, LA, U.S.A.; 2Weill Cornell Medical College, New York, NY, U.S.A. Abstract. Background/Aim: Evaluations of efficacy of distance less than 30 miles from treatment Center, diagnosis treatment modality in analyses on patients with acute myeloid and treatment at same facility, were independently associated leukemia (AML) often combine chemotherapy and stem cell with worse survival. Conclusion: Survival analysis of AML in transplantation (SCT). To account for the effect of SCT and the National Cancer Database showed multiple factors to be determine the impact of chemotherapy alone, the National independently associated with survival. Outcomes based on Cancer Data Base from 1998-2011 was analyzed. Patients and treatment suggest an improved survival when utilizing Methods: Patients with AML from 1998-2011 aged 18-64 years chemotherapy and SCT as the primary treatment modality. were included. Chi-square analysis was used to assess the association between treatment and factors investigated. The The American Cancer Society estimated there were Kaplan–Meier method was used to assess overall survival. approximately 20,830 new cases and 10,460 deaths from Log-rank methods were used to determine factors significant acute myeloid leukemia (AML) in 2015 (1). AML is for survival. Multivariable Cox regression analysis was used to generally a disease of older people and uncommon before determine the effect of chemotherapy alone, and both the age of 45 years. -

Nanocarriers As Magic Bullets in the Treatment of Leukemia
nanomaterials Review Nanocarriers as Magic Bullets in the Treatment of Leukemia 1, 2, 1 2 Mohammad Houshmand y , Francesca Garello y , Paola Circosta , Rachele Stefania , Silvio Aime 2, Giuseppe Saglio 1 and Claudia Giachino 1,* 1 Department of Clinical and Biological Sciences, University of Torino, 10043 Torino, Italy; [email protected] (M.H.); [email protected] (P.C.); [email protected] (G.S.) 2 Molecular and Preclinical Imaging Centres, Department of Molecular Biotechnology and Health Sciences, University of Torino, 10126 Torino, Italy; [email protected] (F.G.); [email protected] (R.S.); [email protected] (S.A.) * Correspondence: [email protected] These authors contributed equally to this work. y Received: 23 December 2019; Accepted: 1 February 2020; Published: 6 February 2020 Abstract: Leukemia is a type of hematopoietic stem/progenitor cell malignancy characterized by the accumulation of immature cells in the blood and bone marrow. Treatment strategies mainly rely on the administration of chemotherapeutic agents, which, unfortunately, are known for their high toxicity and side effects. The concept of targeted therapy as magic bullet was introduced by Paul Erlich about 100 years ago, to inspire new therapies able to tackle the disadvantages of chemotherapeutic agents. Currently, nanoparticles are considered viable options in the treatment of different types of cancer, including leukemia. The main advantages associated with the use of these nanocarriers summarized as follows: i) they may be designed to target leukemic cells selectively; ii) they invariably enhance bioavailability and blood circulation half-life; iii) their mode of action is expected to reduce side effects. -

Mtorc1/2 Inhibition Preserves Ovarian Function and Fertility During Genotoxic Chemotherapy
mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy Kara N. Goldmana, Devon Chenetteb, Rezina Arjub, Francesca E. Duncanc, David L. Keefea, Jamie A. Grifoa, and Robert J. Schneiderb,d,1 aDivision of Reproductive Endocrinology and Infertility, Department of Obstetrics and Gynecology, New York University School of Medicine, New York,NY 10016; bDepartment of Microbiology, New York University School of Medicine, New York, NY 10016; cDepartment of Obstetrics and Gynecology, Feinberg School of Medicine, Northwestern University, Chicago, IL 60611; and dPerlmutter Cancer Center, New York University School of Medicine, New York, NY 10016 Edited by Nahum Sonenberg, McGill University, Montreal, QC, Canada, and approved February 8, 2017 (received for review October 17, 2016) The ovary contains oocytes within immature (primordial) follicles pathway, leading to primordial follicle activation and follicular that are fixed in number at birth. Activation of follicles within this “burnout” (6, 7). fixed pool causes an irreversible decline in reproductive capacity, Ovarian folliculogenesis initiates from the primordial follicle known as the ovarian reserve, until menopause. Premenopausal stage, where an oocyte arrested in prophase of meiosis I and women undergoing commonly used genotoxic (DNA-damaging) surrounded by a single layer of squamous granulosa cells is ac- chemotherapy experience an accelerated loss of the ovarian tivated to grow and transition to a primary follicle, secondary reserve, leading to subfertility and infertility. Therefore, there is follicle, and then ultimately a preovulatory antral follicle (Fig. considerable interest but little effective progress in preserving S1). Most oocytes within the ovary exist in a quiescent state ovarian function during chemotherapy. Here we show that block- within primordial follicles, relatively resistant to antimitotic and ing the kinase mammalian/mechanistic target of rapamycin genotoxic agents (8, 9). -

Current Trends in Cancer Immunotherapy
biomedicines Review Current Trends in Cancer Immunotherapy Ivan Y. Filin 1 , Valeriya V. Solovyeva 1 , Kristina V. Kitaeva 1, Catrin S. Rutland 2 and Albert A. Rizvanov 1,3,* 1 Institute of Fundamental Medicine and Biology, Kazan Federal University, 420008 Kazan, Russia; [email protected] (I.Y.F.); [email protected] (V.V.S.); [email protected] (K.V.K.) 2 Faculty of Medicine and Health Science, University of Nottingham, Nottingham NG7 2QL, UK; [email protected] 3 Republic Clinical Hospital, 420064 Kazan, Russia * Correspondence: [email protected]; Tel.: +7-905-316-7599 Received: 9 November 2020; Accepted: 16 December 2020; Published: 17 December 2020 Abstract: The search for an effective drug to treat oncological diseases, which have become the main scourge of mankind, has generated a lot of methods for studying this affliction. It has also become a serious challenge for scientists and clinicians who have needed to invent new ways of overcoming the problems encountered during treatments, and have also made important discoveries pertaining to fundamental issues relating to the emergence and development of malignant neoplasms. Understanding the basics of the human immune system interactions with tumor cells has enabled new cancer immunotherapy strategies. The initial successes observed in immunotherapy led to new methods of treating cancer and attracted the attention of the scientific and clinical communities due to the prospects of these methods. Nevertheless, there are still many problems that prevent immunotherapy from calling itself an effective drug in the fight against malignant neoplasms. This review examines the current state of affairs for each immunotherapy method, the effectiveness of the strategies under study, as well as possible ways to overcome the problems that have arisen and increase their therapeutic potentials. -

Introduction to Chemotherapy
2/21/2017 Principles of Chemotherapy EUGENE R. PRZESPOLEWSKI, PHARM.D. BCOP THE JONAH CENTER FOR ONCOLOGY AND HEMATOLOGY ERIE COUNTY MEDICAL CENTER Biology 101 2 Cancer is a complex disease caused by genetic and epigenetic mutations Simply, it is only unregulated cell division “Traditional” chemotherapy highjacks mechanisms of mitosis Understanding chemotherapy needs understanding of Biology 101* * Of course it gets complicated Chemotherapy 3 Merriam-Webster: Chemotherapy: noun: che・mo・ther・a・py Medical: The use of chemical agents in the treatment or control of disease (such as cancer) or mental illness Word originated around 1910 by Paul Ehrlich Developed the first treatment for syphilis, antiserum for diphtheria (Nobel prize in 1908) He also developed the concept of “magic bullet” In the world of pharmacology chemotherapy can be used to treat: Infectious disease Cancer 1 2/21/2017 History of Chemotherapy Begins… 4 World War II 5 Nitrogen Mustards were taboo and not used in battle, however Ready to be used (feared Hitler would use when he was pushed) Bomb raid on Bari, Italy on December 2nd, 1943 Sailors exposed had depletion of bone marrow stores and lymph nodes Goodman and Gilman at Yale discovered murine models with lymphomas responded to nitrogen mustard therapy Convinced a surgeon to treat a single NHL patient with a nitrogen mustard Original trial done in 1943, but data kept secret until 1946 The Lesson of the 1940s 6 Nitrogen Mustards: Alkylation of guanine nucleotides in DNA causing inhibition of cell division -
Double Autophagy Stimulation Using Chemotherapy and Mtor Inhibition Combined with Hydroxychloroquine for Autophagy Modulation In
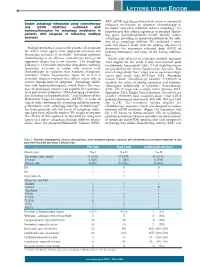
LETTERS TO THE EDITOR AKT- mTOR signaling pathway both serves as a potential Double autophagy stimulation using chemotherapy resistance mechanism to cytotoxic chemotherapy in and mTOR inhibition combined with myeloma10 and when inhibited, induces autophagy,11 we hydroxychloroquine for autophagy modulation in hypothesized that adding rapamycin to standard alkylat- patients with relapsed or refractory multiple ing agent cyclophosphamide would ‘doubly’ induce myeloma autophagy, providing an improved platform for the addi- tion of an autophagy inhibitor. We conducted a safety pilot and phase I study with the primary objective to Multiple myeloma is an incurable plasma cell neoplasm determine the maximum tolerated dose (MTD) of for which novel agents have improved outcomes but hydroxychloroquine and safety of the 4-drug combina- therapeutic resistance is inevitable. Infusional cytotoxic tion. chemotherapy is an effective cytoreductive strategy for Adults with relapsed or refractory multiple myeloma aggressive relapse but is not curative.1 The autophagy were eligible for the study if they had received prior pathway is a lysosome-dependent degradative pathway lenalidomide, bortezomib (Table 1). Full eligibility criteria frequently activated in tumor cells treated with are provided in the Online Supplementary Appendix. This chemotherapy or radiation that mediates therapeutic was a 2-stage study: the 1st stage was an open label single resistance2 (Online Supplementary Figure S1) as it is a center pilot study (July 2011-June 2012; Abramson reversible adaptive response that allows cancer cells to Cancer Center, clinicaltrials.gov identifier: 01396200) to survive therapy-induced apoptosis.3 Autophagy inhibi- establish the safety of adding rapamycin and hydroxy- tion with hydroxychloroquine, which blocks the clear- chloroquine individually to backbone chemotherapy ance of autophagic vesicles,4 can augment the cytotoxici- (n=6). -

Mtor Inhibition Sensitizes Gastric Cancer to Alkylating Chemotherapy in Vivo
ANTICANCER RESEARCH 28 : 3801-3808 (2008) mTOR Inhibition Sensitizes Gastric Cancer to Alkylating Chemotherapy In Vivo DANIEL CEJKA 1, MATTHIAS PREUSSER 2, THORSTEN FUEREDER 1, WOLFGANG SIEGHART 1, JOHANNES WERZOWA 1, SABINE STROMMER 1 and VOLKER WACHECK 1 1Section of Experimental Oncology/Molecular Pharmacology, Department of Clinical Pharmacology, and 2Department of Internal Medicine I, Medical University Vienna, Waehringer Guertel 18-20, 1090 Vienna, Austria Abstract. Background: Gastric cancer is a highly high dose of cyclophosphamide shows synergistic antitumor chemoresistant tumor. Previous studies suggest that cancer activity against gastric cancer in vivo. In potential future cells can be sensitized to standard chemotherapy, and clinical trials, the toxicity of cyclophosphamide in combination especially alkylating agents, by inhibition of mammalian target regimens with everolimus deserves careful evaluation. of rapamycin (mTOR) signaling. The work presented here shows that the mTOR inhibitor everolimus, in combination The mammalian target of rapamycin (mTOR) pathway has with cyclophosphamide, exhibit s synergistic antitumor activity become a major focus of preclinical and clinical cancer in gastric cancer xenografts. Materials and Methods: research (1). Rapamycin inhibits the kinase activity of mTOR, Treatment with everolimus at the minimal effective dose was which has been shown to result in G1 arrest, apoptosis or studied in combination with cyclophosphamide at maximum autophagy, depending on cell type studied (2-4). In gastric tolerated dose in a human gastric cancer severe combined cancer, mTOR activity and components of the mTOR immunodeficient (SCID) mouse xenograft model. Besides signaling network including PTEN, 4E-BP1 and eIF-4 are tumor size, biomarker expression for proliferation (Ki-67), deregulated and correlate with progression of disease, hypoxia (HIF-1α), apoptosis (activated caspase 3), metastasis, and inferior survival (5-9).