Linnaea Borealis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Checklist of Common Native Plants the Diversity of Acadia National Park Is Refl Ected in Its Plant Life; More Than 1,100 Plant Species Are Found Here
National Park Service Acadia U.S. Department of the Interior Acadia National Park Checklist of Common Native Plants The diversity of Acadia National Park is refl ected in its plant life; more than 1,100 plant species are found here. This checklist groups the park’s most common plants into the communities where they are typically found. The plant’s growth form is indicated by “t” for trees and “s” for shrubs. To identify unfamiliar plants, consult a fi eld guide or visit the Wild Gardens of Acadia at Sieur de Monts Spring, where more than 400 plants are labeled and displayed in their habitats. All plants within Acadia National Park are protected. Please help protect the park’s fragile beauty by leaving plants in the condition that you fi nd them. Deciduous Woods ash, white t Fraxinus americana maple, mountain t Acer spicatum aspen, big-toothed t Populus grandidentata maple, red t Acer rubrum aspen, trembling t Populus tremuloides maple, striped t Acer pensylvanicum aster, large-leaved Aster macrophyllus maple, sugar t Acer saccharum beech, American t Fagus grandifolia mayfl ower, Canada Maianthemum canadense birch, paper t Betula papyrifera oak, red t Quercus rubra birch, yellow t Betula alleghaniesis pine, white t Pinus strobus blueberry, low sweet s Vaccinium angustifolium pyrola, round-leaved Pyrola americana bunchberry Cornus canadensis sarsaparilla, wild Aralia nudicaulis bush-honeysuckle s Diervilla lonicera saxifrage, early Saxifraga virginiensis cherry, pin t Prunus pensylvanica shadbush or serviceberry s,t Amelanchier spp. cherry, choke t Prunus virginiana Solomon’s seal, false Maianthemum racemosum elder, red-berried or s Sambucus racemosa ssp. -

Linnaeus at Home
NATURE-BASED ACTIVITIES FOR PARENTS LINNAEUS 1 AT HOME A GuiDE TO EXPLORING NATURE WITH CHILDREN Acknowledgements Written by Joe Burton Inspired by Carl Linnaeus With thanks to editors and reviewers: LINNAEUS Lyn Baber, Melissa Balzano, Jane Banham, Sarah Black, Isabelle Charmantier, Mark Chase, Maarten Christenhusz, Alex Davey, Gareth Dauley, AT HOME Zia Forrai, Jon Hale, Simon Hiscock, Alice ter Meulen, Lynn Parker, Elizabeth Rollinson, James Rosindell, Daryl Stenvoll-Wells, Ross Ziegelmeier Share your explorations @LinneanLearning #LinnaeusAtHome Facing page: Carl Linnaeus paper doll, illustrated in 1953. © Linnean Society of London 2019 All rights reserved. No part of this publication may be reproduced, stored in a retrival system or trasmitted in any form or by any means without the prior consent of the copyright owner. www.linnean.org/learning “If you do not know Introduction the names of things, the knowledge of them is Who was Carl Linnaeus? Contents Pitfall traps 5 lost too” Carl Linnaeus was one of the most influential scientists in the world, - Carl Linnaeus A bust of ‘The Young Linnaeus’ by but you might not know a lot about him. Thanks to Linnaeus, we Bug hunting 9 Anthony Smith (2007). have a naming system for all species so that we can understand how different species are related and can start to learn about the origins Plant hunting 13 of life on Earth. Pond dipping 17 As a young man, Linnaeus would study the animals, plants, Bird feeders 21 minerals and habitats around him. By watching the natural world, he began to understand that all living things are adapted to their Squirrel feeders 25 environments and that they can be grouped together by their characteristics (like animals with backbones, or plants that produce Friendly spaces 29 spores). -

Ornamental Garden Plants of the Guianas Pt. 2
Surinam (Pulle, 1906). 8. Gliricidia Kunth & Endlicher Unarmed, deciduous trees and shrubs. Leaves alternate, petiolate, odd-pinnate, 1- pinnate. Inflorescence an axillary, many-flowered raceme. Flowers papilionaceous; sepals united in a cupuliform, weakly 5-toothed tube; standard petal reflexed; keel incurved, the petals united. Stamens 10; 9 united by the filaments in a tube, 1 free. Fruit dehiscent, flat, narrow; seeds numerous. 1. Gliricidia sepium (Jacquin) Kunth ex Grisebach, Abhandlungen der Akademie der Wissenschaften, Gottingen 7: 52 (1857). MADRE DE CACAO (Surinam); ACACIA DES ANTILLES (French Guiana). Tree to 9 m; branches hairy when young; poisonous. Leaves with 4-8 pairs of leaflets; leaflets elliptical, acuminate, often dark-spotted or -blotched beneath, to 7 x 3 (-4) cm. Inflorescence to 15 cm. Petals pale purplish-pink, c.1.2 cm; standard petal marked with yellow from middle to base. Fruit narrowly oblong, somewhat woody, to 15 x 1.2 cm; seeds up to 11 per fruit. Range: Mexico to South America. Grown as an ornamental in the Botanic Gardens, Georgetown, Guyana (Index Seminum, 1982) and in French Guiana (de Granville, 1985). Grown as a shade tree in Surinam (Ostendorf, 1962). In tropical America this species is often interplanted with coffee and cacao trees to shade them; it is recommended for intensified utilization as a fuelwood for the humid tropics (National Academy of Sciences, 1980; Little, 1983). 9. Pterocarpus Jacquin Unarmed, nearly evergreen trees, sometimes lianas. Leaves alternate, petiolate, odd- pinnate, 1-pinnate; leaflets alternate. Inflorescence an axillary or terminal panicle or raceme. Flowers papilionaceous; sepals united in an unequally 5-toothed tube; standard and wing petals crisped (wavy); keel petals free or nearly so. -

Nomenclatural Studies Toward a World List of Diptera Genus-Group Names
Nomenclatural studies toward a world list of Diptera genus-group names. Part V Pierre-Justin-Marie Macquart Evenhuis, Neal L.; Pape, Thomas; Pont, Adrian C. DOI: 10.11646/zootaxa.4172.1.1 Publication date: 2016 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Evenhuis, N. L., Pape, T., & Pont, A. C. (2016). Nomenclatural studies toward a world list of Diptera genus- group names. Part V: Pierre-Justin-Marie Macquart. Magnolia Press. Zootaxa Vol. 4172 No. 1 https://doi.org/10.11646/zootaxa.4172.1.1 Download date: 02. Oct. 2021 Zootaxa 4172 (1): 001–211 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Monograph ZOOTAXA Copyright © 2016 Magnolia Press ISSN 1175-5334 (online edition) http://doi.org/10.11646/zootaxa.4172.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:22128906-32FA-4A80-85D6-10F114E81A7B ZOOTAXA 4172 Nomenclatural Studies Toward a World List of Diptera Genus-Group Names. Part V: Pierre-Justin-Marie Macquart NEAL L. EVENHUIS1, THOMAS PAPE2 & ADRIAN C. PONT3 1 J. Linsley Gressitt Center for Entomological Research, Bishop Museum, 1525 Bernice Street, Honolulu, Hawaii 96817-2704, USA. E-mail: [email protected] 2 Natural History Museum of Denmark, Universitetsparken 15, 2100 Copenhagen, Denmark. E-mail: [email protected] 3Oxford University Museum of Natural History, Parks Road, Oxford OX1 3PW, UK. E-mail: [email protected] Magnolia Press Auckland, New Zealand Accepted by D. Whitmore: 15 Aug. 2016; published: 30 Sept. 2016 Licensed under a Creative Commons Attribution License http://creativecommons.org/licenses/by/3.0 NEAL L. -

Ad Hoc Referees Committee for This Issue Thomas Dirnböck
COMITATO DI REVISIONE PER QUESTO NUMERO – Ad hoc referees committee for this issue Thomas Dirnböck Umweltbundesamt GmbH Studien & Beratung II, Spittelauer Lände 5, 1090 Wien, Austria Marco Kovac Slovenian Forestry Institute, Vecna pot 2, 1000 Ljubljana, Slovenija Susanna Nocentini Università degli Studi di Firenze, DISTAF, Via S. Bonaventura 13, 50145 Firenze Ralf Ohlemueller Department of Biology, University of York, PO Box 373, York YO10 5YW, UK Sandro Pignatti Orto Botanico di Roma, Dipartimento di Biologia Vegetale, L.go Cristina di Svezia, 24, 00165 Roma Stergios Pirintsos Department of Biology, University of Crete, P.O.Box 2208, 71409 Heraklion, Greece Matthias Plattner Hintermann & Weber AG, Oeko-Logische Beratung Planung Forschung, Hauptstrasse 52, CH-4153 Reinach Basel Arne Pommerening School of Agricultural & Forest Sciences, University of Wales, Bangor, Gwynedd LL57 2UW, DU/ UK Roberto Scotti Università degli Studi di Sassari, DESA, Nuoro branch, Via C. Colombo 1, 08100 Nuoro Franz Starlinger Forstliche Bundesversuchsanstalt Wien, A 1131 Vienna, Austria Silvia Stofer Eidgenössische Forschungsanstalt für Wald, Schnee und Landschaft – WSL, Zürcherstrasse 111, CH-8903 Birmensdorf, Switzerland Norman Woodley Systematic Entomology Lab-USDA , c/o Smithsonian Institution NHB-168 , O Box 37012 Washington, DC 20013-7012 CURATORI DI QUESTO NUMERO – Editors Marco Ferretti, Bruno Petriccione, Gianfranco Fabbio, Filippo Bussotti EDITORE – Publisher C.R.A. - Istituto Sperimentale per la Selvicoltura Viale Santa Margherita, 80 – 52100 Arezzo Tel.. ++39 0575 353021; Fax. ++39 0575 353490; E-mail:[email protected] Volume 30, Supplemento 2 - 2006 LIST OF CONTRIBUTORS C.R.A.A - ISTITUTO N SPERIMENTALE N A PER LA LSELVICOLTURA I (in alphabetic order) Allegrini, M. C. -

Wildlife Viewing
Wildlife Viewing Common Yukon roadside flowers © Government of Yukon 2019 ISBN 987-1-55362-830-9 A guide to common Yukon roadside flowers All photos are Yukon government unless otherwise noted. Bog Laurel Cover artwork of Arctic Lupine by Lee Mennell. Yukon is home to more than 1,250 species of flowering For more information contact: plants. Many of these plants Government of Yukon are perennial (continuously Wildlife Viewing Program living for more than two Box 2703 (V-5R) years). This guide highlights Whitehorse, Yukon Y1A 2C6 the flowers you are most likely to see while travelling Phone: 867-667-8291 Toll free: 1-800-661-0408 x 8291 by road through the territory. Email: [email protected] It describes 58 species of Yukon.ca flowering plant, grouped by Table of contents Find us on Facebook at “Yukon Wildlife Viewing” flower colour followed by a section on Yukon trees. Introduction ..........................2 To identify a flower, flip to the Pink flowers ..........................6 appropriate colour section White flowers .................... 10 and match your flower with Yellow flowers ................... 19 the pictures. Although it is Purple/blue flowers.......... 24 Additional resources often thought that Canada’s Green flowers .................... 31 While this guide is an excellent place to start when identi- north is a barren landscape, fying a Yukon wildflower, we do not recommend relying you’ll soon see that it is Trees..................................... 32 solely on it, particularly with reference to using plants actually home to an amazing as food or medicines. The following are some additional diversity of unique flora. resources available in Yukon libraries and bookstores. -

A Computerized Annotated Bibliography of Partial-Cutting Methods in the Pacific Northwest (1995 Update)
FRDA FORESTRY HANDBOOK 010 PARTCUTS: A Computerized Annotated Bibliography of Partial-cutting Methods in the Pacific Northwest (1995 Update) ISSN 0835 1929 MARCH 1995 CANADA-BRITISH COLUMBIA PARTNERSHIP AGREEMENT ON FOREST RESOURCE DEVELOPMENT: FRDA II PARTCUTS: A Computerized Annotated Bibliography of Partial-cutting Methods in the Pacific Northwest (1995 Update) by Patrick W. Daigle Compiler and Editor Research Branch BC Ministry of Forests 31 Bastion Square Victoria, B.C. V8W 3E7 March 1995 CANADA-BRITISH COLUMBIA PARTNERSHIP AGREEMENT ON FOREST RESOURCE DEVELOPMENT: FRDA II Funding for this publication was provided by the Canada-British Columbia Partnership Agreement on Forest Resource Development: FRDA II - a four year (1991-95) $200 million program cost-shared equally by the federal and provincial governments. Canadian Cataloguing in Publication Data Daigle, Patrick W. PARTCUTS: a computerized annotated bibliography of partial-cutting methods in the Pacific Northwest (1995 Update) (FRDA handbook, ISSN 0835-1929 ; 010) "Canada-British Columbia Partnership Agreement on Forest Resource Development: FRDA II." Co-published by BC Ministry of Forests. Includes bibliographical references: p. ISBN 0-7726-1658-2 1. Information storage and retrieval systems- Forestry - Handbooks, manuals, etc. 2. Forests and forestry - research - Northwest, Pacific - Data processing - Handbooks, manuals, etc. 3. Forests and forestry - Northwest, Pacific - Bibliography. 4. Forest management - Northwest, Pacific - Bibliography. I. Comeau, P.G., 1954 - .II. Canada. Forestry Canada. III. Canada-British Columbia Partnership Agreement on Forest Resource Development: FRDA II. IV. British Columbia Ministry of Forests. V. Title. VI. Series. SD381.5.D34 1992 025.06'634953 C92-092374-7 1995 Government of Canada, Province of British Columbia This is a joint publication of Forestry Canada and the British Columbia Ministry of Forests. -

Twinflower (Linnaea Borealis L.) – Plant Species of Potential Medicinal Properties
From Botanical to Medical Research Vol. 63 No. 3 2017 DOI: 10.1515/hepo-2017-0019 REVIEW PAPER Twinflower (Linnaea borealis L.) – plant species of potential medicinal properties BARBARA THIEM1* ELISABETH BUK-BERGE2 1Department of Pharmaceutical Botany and Plant Biotechnology Poznań University of Medical Sciences Św. Marii Magdaleny 14 61-861 Poznań, Poland 2The Norwegian Ministry of Education and Research** Postbox 8119 Dep. 0032 Oslo, Norway *corresponding author: phone: +4861 6687851, e-mail: [email protected] Summary Twinflower (Linnaea borealis L.) is a widespread circumboreal plant species belonging to Linnaeaceae family (previously Caprifoliaceae). L. borealis commonly grows in taiga and tundra. In some countries in Europe, including Poland, twinflower is protected as a glacial relict. Chemical composition of this species is not well known, however in folk medicine of Scandinavian countries, L. borealis has a long tradition as a cure for skin diseases and rheumatism. It is suggested that twinflower has potential medicinal properties. The new study on lead secondary metabolites responsible for biological activity are necessary. This short review summarizes very sparse knowledge on twinflower: its biology, distribution, conservation status, chemical constituents, and describes the role of this plant in folk tradition of Scandinavian countries. Key words: Linnaea borealis, botanical description, distribution, secondary metabolites, folk medicine INTRODUCTION of the Linnaea genus was provided by Christen- husz in 2013 [3]. The genusLinnaea was reviewed Linnaea borealis L. (eng. twinflower; pol. zimoziół and expanded to include the genera: Abelia, Di- północny, Linnea północna; nor. Nårislegras, Flis- pelta, Diabelia, Kolkwitzia and Vesalea. In gen- megras) belongs to Linnaeaceae family, formerly eral, in the new depiction this taxon consist of 16 to Caprifoliaceae [1, 2]. -

2009) Summary Report: Tanacross Shaded Fuelbreak AA39, 9/29/09
2009 SUMMARY REPORT R.R. Jandt 9/29/09 Tanacross Shaded Fuel Break AA39 Fuel Reduction Project Local residents, working with the village council and Alaska Fire Service, received federal funding to reduce the fire risk and hazard to private residential structures by modifying fuel structure and continuity of 66 acres around the community of Tanacross. Treatment was intended to produce a more open stand to slow the rate of spread and intensity of an accidental fire. At the same time, residents wanted to minimize the visual and ecological impact of the shaded fuel break by using hand Figure 1. Hand crew thinning spruce stand and removing crews to treat the area instead of ladder fuels around Tanacross. heavy equipment (Fig. 1). Vegetation Cover Three permanent transects, measuring 30m x 3m, were established in 2001 to monitor changes in understory vegetation cover. Though vegetation composition differed slightly between transects, pre-treatment (2001) understory cover was dominated by heath shrub, such as low-bush cranberry (Vaccinium vitis-idaea) and live feather moss with some tall willows (Salix bebbiana, primarily). Other common species pre-treatment included crowberry (Empetrum nigrum), twinflower (Linnaea borealis), and bastard toadflax (Geocaulon lividum). White spruce (Picea glauca) was the dominant overstory tree. Transects were monitored from 2002-2004 and in 2009 to assess changes in cover type. The most notable change was loss of viability of the feather moss cover on the forest floor in the first two summers following the treatment (Fig. 2, 3). Live moss was almost 50% of the substrate (ground cover) in 2001, whereas by 2003 less than 5% was recorded as live and 22% of the substrate cover was dead feather moss. -

Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -
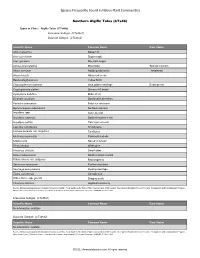
Algific Talus (Cts46)
Species Frequently Found in Native Plant Communities Southern Algific Talus (CTs46) Types in Class: Algific Talus (CTs46a) Limestone Subtype (CTs46a1) Dolomite Subtype (CTs46a2) Scientific Name Column1 Common Name Rare Status Abies balsamea Balsam fir Acer saccharum Sugar maple Acer spicatum Mountain maple Adoxa moschatellina Moschatel Special Concern Allium cernuum Nodding wild onion Threatened Arabis hirsuta Hairy rock cress Betula alleghaniensis Yellow birch Chrysosplenium iowense Iowa golden saxifrage Endangered Cryptogramma stelleri Slender cliff brake Cystopteris bulbifera Bulblet fern Dicentra cucullaria Dutchman's breeches Enemion biternatum False rue anemone Gymnocarpium robertianum Northern oak fern Impatiens spp. touch-me-not Impatiens capensis Spotted touch-me-not Impatiens pallida Pale touch-me-not Laportea canadensis Wood nettle Linnaea borealis var. longiflora Twinflower Mertensia paniculata Panicled bluebells Mitella nuda Naked miterwort Pinus strobus White pine Rhamnus alnifolia Dwarf alder Ribes hudsonianum Northern black currant Rubus idaeus var. strigosus Red raspberry Sambucus racemosa Red-berried elder Saxifraga pensylvanica Swamp saxifrage Taxus canadensis Canada yew Urtica dioica ssp. gracilis Stinging nettle Viburnum trilobum Highbush cranberry Source: Minnesota Department of Natural Resources (2005). Field Guide to the Native Plant Communities of Minnesota: The Eastern Broadleaf Forest Province. Ecological Land Classification Program, Minnesota County Biological Survey, and Natural Heritage and Nongame Research Program. MNDNR St. Paul, MN. Limestone Subtype (CTs46a1) Scientific Name Column1 Common Name Rare Status No information available Dolomite Subtype (CTs46a2) Scientific Name Column1 Common Name Rare Status No information available Source: Minnesota Department of Natural Resources (2005). Field Guide to the Native Plant Communities of Minnesota: The Eastern Broadleaf Forest Province. Ecological Land Classification Program, Minnesota County Biological Survey, and Natural Heritage and Nongame Research Program. -

Conserving Plant Diversity in New England
CONSERVING PLANT DIVERSITY IN NEW ENGLAND A COLLABORATION OF CONSERVING PLANT DIVERSITY IN NEW ENGLAND A COLLABORATION OF AUTHORS Mark Anderson Director of Conservation Science, Eastern United States, The Nature Conservancy Michael Piantedosi Director of Conservation, Native Plant Trust William Brumback Director of Conservation Emeritus, Native Plant Trust MAP PRODUCTION Arlene Olivero WEB TOOL Melissa Clark DESIGN Rachel Wolff-Lander Kate Wollensak Freeborn The authors wish to thank the six state Natural Heritage programs for sharing their data and for their support. ©2021 Published June 2021 © Peter James CONTENTS EXECUTIVE SUMMARY ES-1 PART ONE: CONSERVING PLANT DIVERSITY 1-1 Background 1-2 • Plant Diversity and Resilience 1-2 • Global Strategy for Plant Conservation and Global Deal for Nature 1-7 • Secured Lands and GAP Status 1-9 • New England Flora and Rare Taxa 1-11 • Threats to Plant Diversity in New England 1-14 • Conservation Actions to Counter Threats to Plant Diversity 1-17 Conservation of Habitats and Important Plant Areas 1-21 Introduction 1-21 • Terminology 1-21 • Overview and Methods 1-22 Conservation of Habitats: Progress Toward Global and Regional Goals 1-26 • Matrix Forests 1-26 • Wetlands 1-30 • Patch-forming Habitats 1-33 • Risk of Conversion 1-36 Conservation of Important Plant Areas (IPAs) 1-37 • Definition and Location of IPAs 1-37 • Conservation Status and Progress Toward Goals 1-40 • Representation of Habitats in the IPAs 1-42 Conservation of Threatened Species 1-48 • Threatened Plants Conserved in situ 1-48 • Threatened Plants Conserved in ex situ Collections 1-49 Results and Recommendations 1-58 i CONTENTS continued PART TWO: STATUS REPORT AND MAPS 2-1 Overview 2-4 New England’s Terrestrial Habitats 2-7 Map Page Layout 2-13 Upland Habitats: Matrix Forest 2-16 Upland Habitats: Patch-forming Habitats 2-53 Wetland Habitats 2-80 PART THREE: SUPPORTING MATERIAL 3-1 Bibliography 3-2 Appendices 3-11 1.