Usher Syndrome, Types IF and IIIA
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
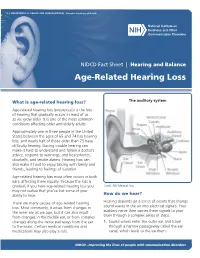
Age-Related Hearing Loss
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES ∙ National Institutes of Health NIDCD Fact Sheet | Hearing and Balance Age-Related Hearing Loss What is age-related hearing loss? The auditory system Age-related hearing loss (presbycusis) is the loss of hearing that gradually occurs in most of us as we grow older. It is one of the most common conditions affecting older and elderly adults. Approximately one in three people in the United States between the ages of 65 and 74 has hearing loss, and nearly half of those older than 75 have difficulty hearing. Having trouble hearing can make it hard to understand and follow a doctor’s advice, respond to warnings, and hear phones, doorbells, and smoke alarms. Hearing loss can also make it hard to enjoy talking with family and friends, leading to feelings of isolation. Age-related hearing loss most often occurs in both ears, affecting them equally. Because the loss is gradual, if you have age-related hearing loss you Credit: NIH Medical Arts may not realize that you’ve lost some of your ability to hear. How do we hear? There are many causes of age-related hearing Hearing depends on a series of events that change loss. Most commonly, it arises from changes in sound waves in the air into electrical signals. Your the inner ear as we age, but it can also result auditory nerve then carries these signals to your from changes in the middle ear, or from complex brain through a complex series of steps. changes along the nerve pathways from the ear 1. -

Novel Association of Hypertrophic Cardiomyopathy, Sensorineural Deafness, and a Mutation in Unconventional Myosin VI (MYO6)
309 LETTER TO JMG J Med Genet: first published as 10.1136/jmg.2003.011973 on 1 April 2004. Downloaded from Novel association of hypertrophic cardiomyopathy, sensorineural deafness, and a mutation in unconventional myosin VI (MYO6) S A Mohiddin, Z M Ahmed, A J Griffith, D Tripodi, T B Friedman, L Fananapazir, R J Morell ............................................................................................................................... J Med Genet 2004;41:309–314. doi: 10.1136/jmg.2003.011973 amilial hypertrophic cardiomyopathy (FHC) is typically Key points characterised by left ventricular hypertrophy, diastolic Fdysfunction, and hypercontractility, and is often asso- ciated with disabling symptoms, arrhythmias, and sudden N Familial hypertrophic cardiomyopathy (FHC) is typi- death.1 FHC shows both non-allelic and allelic genetic cally confined to a cardiac phenotype and is caused by heterogeneity, and results from any one of more than 100 mutations in genes encoding sarcomeric proteins. mutations in genes encoding sarcomeric proteins.2 Identified Occasionally FHC may be one component of a genes include those encoding b myosin heavy chain, the hereditary multisystem disorder. myosin regulatory and essential light chains, myosin bind- N Sensorineural hearing loss is genetically heteroge- ing protein C, troponin I, troponin C, a cardiac actin, and neous. Mutations in the MYO6 gene, encoding 23 titin. The FHC phenotype is characterised by hypertrophy, unconventional myosin VI, have been found to cause myocyte disarray and fibrosis, and results from the dominant non-syndromic sensorineural hearing loss—that is, negative expression of one of these (mainly missense) sensorineural hearing loss in the absence of any other mutations. The resulting sarcomeric dysfunction leads related clinical features. ultimately, through mechanisms that remain obscure, to pathological left ventricular remodelling. -

Clinical Genetics and the Hutterite Brethren
Clinical Genetics and the Hutterite Brethren: What have we learned in the new millenium? Or: A Micheil Innes MD FRCPC FCCMG Adapted from: Medical Genetics Grand Rounds January 2013 History and Population Hutterite Population Today >40 000 in AB, 30000 MB, ND, SD 1593-1770 1874-1879 25000 Transylvania 1256 migrated to American Prairies 20000 15000 World War I 10000 1565-1592 1770 - 1870 Migration to Canada Moravia5000 Ukraine 0 1500s 1520 1540 1550 1570 1580 1590 1610 1620 1680 1750 1760 1840 1860 1890 1900 1950 1975 1990 Tyrolean Alps Why Identify Genes in this Population? • Direct Benefits to • Benefit to Larger Patients/Families population – Non-invasive – Most of these disorders diagnostic test are not confined to this – Carrier test (*marriage population restrictions) – May allow for diagnosis – ?Prenatal testing of atypical cases – Enhanced understanding of – Expand basic science disease may facilitate and clinical knowledge management or treatment Initial Presentations May be Non-Specific Highlighting Importance of Careful Syndrome Delineation and Early Genetic Diagnosis • Hearing Loss – Autosomal recessive non-syndromic hearing loss (> 2loci) – Usher syndrome (> 2loci) – HDR syndrome • Cerebellar Ataxia – Joubert syndrome – DES syndrome – DCMA syndrome – CASS syndrome • Muscular Dystrophy/ High CK – LGMD2H – LGDM2I – AR EDMD – Myopathy with CPEO – Microcephaly with Chorea Genetic services and the Hutterites Religion/Culture • Has posed little barrier overall • Very accepting of medical care and technology • Although they believe that God plays a day to day role in guiding their lives, most couples accept genetic explanations for their children’s disorders • Some leuts and individual colonies are more conservative than others • Colony leader is clearly the Minister • Who speaks for the overall community when it comes to community wide issues? – e.g. -

Splicing-Correcting Therapeutic Approaches for Retinal Dystrophies: Where Endogenous Gene Regulation and Specificity Matter
New Developments Splicing-Correcting Therapeutic Approaches for Retinal Dystrophies: Where Endogenous Gene Regulation and Specificity Matter Niccolo` Bacchi,1 Simona Casarosa,1,2 and Michela A. Denti1,3 1Centre for Integrative Biology (CIBIO) - University of Trento, Trento, Italy 2Neuroscience Institute - National Research Council (CNR), Pisa, Italy 3Neuroscience Institute - National Research Council (CNR), Padova, Italy Correspondence: Simona Casarosa, Splicing is an important and highly regulated step in gene expression. The ability to modulate Centre for Integrative Biology it can offer a therapeutic option for many genetic disorders. Antisense-mediated splicing- (CIBIO) - University of Trento, Via correction approaches have recently been successfully exploited for some genetic diseases, Sommarive 9, 38123 Trento, Italy; and are currently demonstrating safety and efficacy in different clinical trials. Their [email protected]. application for the treatment of retinal dystrophies could potentially solve a vast panel of Michela A. Denti, Centre for Inte- grative Biology (CIBIO) - University cases, as illustrated by the abundance of mutations that could be targeted and the versatility of ofTrento,ViaSommarive9,38123 the technique. In this review, we will give an insight of the different therapeutic strategies, Trento, Italy; focusing on the current status of their application for retinal dystrophies. [email protected]. Keywords: splicing correction, antisense oligonucleotides, retinal dystrophy, gene therapy SC and MAD contributed equally to the work presented here and should therefore be regarded as equivalent authors. Submitted: April 8, 2014 Accepted: April 11, 2014 Citation: Bacchi N, Casarosa S, Denti MA. Splicing-correcting therapeutic approaches for retinal dystrophies: where endogenous gene regulation and specificity matter. Invest Oph- thalmol Vis Sci. -
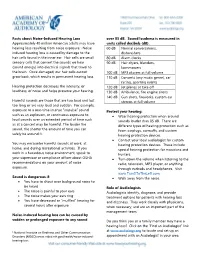
Facts About Noise-Induced Hearing Loss Over 85 Db
Facts about Noise-Induced Hearing Loss over 85 dB. Sound loudness is measured in Approximately 40 million American adults may have units called decibels (dB). hearing loss resulting from noise exposure.1 Noise- 60 dB Normal conversations, induced hearing loss is caused by damage to the dishwashers hair cells found in the inner ear. Hair cells are small 80 dB Alarm clocks sensory cells that convert the sounds we hear 90 dB Hair dryers, blenders, (sound energy) into electrical signals that travel to lawnmowers the brain. Once damaged, our hair cells cannot 100 dB MP3 players at full volume grow back, which results in permanent hearing loss. 110 dB Concerts (any music genre), car racing, sporting events Hearing protection decreases the intensity, or 120 dB Jet planes at take off loudness, of noise and helps preserve your hearing. 130 dB Ambulance, fire engine sirens 140 dB Gun shots, fireworks, custom car Harmful sounds are those that are too loud and last stereos at full volume too long or are very loud and sudden. For example, exposure to a one-time intense “impulse” sound Protect your hearing: such as an explosion, or continuous exposure to • Wear hearing protection when around loud sounds over an extended period of time such sounds louder than 85 dB. There are as at a concert may be harmful. The louder the different types of hearing protection such as sound, the shorter the amount of time you can foam earplugs, earmuffs, and custom safely be around it. hearing protection devices • Contact your local audiologist for custom You may encounter harmful sounds at work, at hearing protection devices. -

Cardiomyopathy Precision Panel Overview Indications
Cardiomyopathy Precision Panel Overview Cardiomyopathies are a group of conditions with a strong genetic background that structurally hinder the heart to pump out blood to the rest of the body due to weakness in the heart muscles. These diseases affect individuals of all ages and can lead to heart failure and sudden cardiac death. If there is a family history of cardiomyopathy it is strongly recommended to undergo genetic testing to be aware of the family risk, personal risk, and treatment options. Most types of cardiomyopathies are inherited in a dominant manner, which means that one altered copy of the gene is enough for the disease to present in an individual. The symptoms of cardiomyopathy are variable, and these diseases can present in different ways. There are 5 types of cardiomyopathies, the most common being hypertrophic cardiomyopathy: 1. Hypertrophic cardiomyopathy (HCM) 2. Dilated cardiomyopathy (DCM) 3. Restrictive cardiomyopathy (RCM) 4. Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) 5. Isolated Left Ventricular Non-Compaction Cardiomyopathy (LVNC). The Igenomix Cardiomyopathy Precision Panel serves as a diagnostic and tool ultimately leading to a better management and prognosis of the disease. It provides a comprehensive analysis of the genes involved in this disease using next-generation sequencing (NGS) to fully understand the spectrum of relevant genes. Indications The Igenomix Cardiomyopathy Precision Panel is indicated in those cases where there is a clinical suspicion of cardiomyopathy with or without the following manifestations: - Shortness of breath - Fatigue - Arrythmia (abnormal heart rhythm) - Family history of arrhythmia - Abnormal scans - Ventricular tachycardia - Ventricular fibrillation - Chest Pain - Dizziness - Sudden cardiac death in the family 1 Clinical Utility The clinical utility of this panel is: - The genetic and molecular diagnosis for an accurate clinical diagnosis of a patient with personal or family history of cardiomyopathy, channelopathy or sudden cardiac death. -

Hearing Loss
Randal W. Swenson, M.D. Joshua G. Yorgason, M.D. David K. Palmer, M.D. Wesley R. Brown, M.D. John E. Butler, M.D. Nancy J. Stevenson, PA-C Justin D. Gull, M.D. ENT SPECIALISTS Kristin G. Hoopes, PA-C www.entslc.com Hearing Loss Approximately one in ten persons in the United may result from blockage of the ear canal (wax), States has some degree of hearing loss. Hearing is from a perforation (hole) in the ear drum, or from measured in decibels (dB), and a hearing level of 0- infection or disease of any of the three middle ear 25 dB is considered normal hearing. Your level is: bones. With a conductive loss only, the patient will never go deaf, but will always be able to hear, either Right ear _______ dB Left ear _______dB with reconstructive ear surgery or by use of a properly fitted hearing aid. Some patients who are Hearing Severity / % Loss not candidates for surgery, may benefit from a new 25 dB (normal).….0% 65dB(Severe)……...60% technology, the Baha (bone-anchored hearing aid). 35 dB (mild)……..15% 75dB(Severe)……...75% When there is a problem with the inner ear or 45 dB (moderate)..30% >85dB (Profound)..>90% nerve of hearing, a sensori-neural hearing loss occurs. This is most commonly from normal aging, Normal speech discrimination is 88-100%. Yours is: is usually worse in high frequencies, and can progress to total deafness. Noise exposure is another Right ear _______ % Left ear_______% common cause of high frequency hearing loss. Patients with sensori-neural hearing loss usually complain of difficulty hearing in loud environments. -

WES Gene Package Multiple Congenital Anomalie.Xlsx
Whole Exome Sequencing Gene package Multiple congenital anomalie, version 5, 1‐2‐2018 Technical information DNA was enriched using Agilent SureSelect Clinical Research Exome V2 capture and paired‐end sequenced on the Illumina platform (outsourced). The aim is to obtain 8.1 Giga base pairs per exome with a mapped fraction of 0.99. The average coverage of the exome is ~50x. Duplicate reads are excluded. Data are demultiplexed with bcl2fastq Conversion Software from Illumina. Reads are mapped to the genome using the BWA‐MEM algorithm (reference: http://bio‐bwa.sourceforge.net/). Variant detection is performed by the Genome Analysis Toolkit HaplotypeCaller (reference: http://www.broadinstitute.org/gatk/). The detected variants are filtered and annotated with Cartagenia software and classified with Alamut Visual. It is not excluded that pathogenic mutations are being missed using this technology. At this moment, there is not enough information about the sensitivity of this technique with respect to the detection of deletions and duplications of more than 5 nucleotides and of somatic mosaic mutations (all types of sequence changes). HGNC approved Phenotype description including OMIM phenotype ID(s) OMIM median depth % covered % covered % covered gene symbol gene ID >10x >20x >30x A4GALT [Blood group, P1Pk system, P(2) phenotype], 111400 607922 101 100 100 99 [Blood group, P1Pk system, p phenotype], 111400 NOR polyagglutination syndrome, 111400 AAAS Achalasia‐addisonianism‐alacrimia syndrome, 231550 605378 73 100 100 100 AAGAB Keratoderma, palmoplantar, -

Preventing Hearing Loss Caused by Chemical (Ototoxicity) and Noise Exposure
Preventing Hearing Loss Caused by Chemical (Ototoxicity) and Noise Exposure Safety and Health Information Bulletin SHIB 03-08-2018 DHHS (NIOSH) Publication No. 2018-124 Introduction Millions of workers are exposed to noise in the workplace every day and when uncontrolled, noise exposure may cause permanent hearing loss. Research demonstrates exposure to certain chemicals, called ototoxicants, may cause hearing loss or balance problems, regardless of noise exposure. Substances including certain pesticides, solvents, and pharmaceuticals that contain ototoxicants can negatively affect how the ear functions, causing hearing loss, and/or affect balance. Source/Copyright: OSHA The risk of hearing loss is increased when workers are exposed to these chemicals while working around elevated noise levels. This combination often results in hearing loss that can be temporary or permanent, depending on the level of noise, the dose of the chemical, and the duration of the exposure. This hearing impairment affects many occupations and industries, from machinists to firefighters. Effects on Hearing Harmful exposure to ototoxicants may occur through inhalation, ingestion, or skin absorption. Health effects caused by ototoxic chemicals vary based on exposure frequency, intensity, duration, workplace exposure to other hazards, and individual factors such as age. Effects may be temporary or permanent, can affect hearing sensitivity and result in a standard threshold shift. Since chemicals can affect central portions of the auditory system (e.g., nerves or nuclei in the central nervous system, the pathways to the brain or in the brain itself), not only do sounds need to be louder to be detected, but also they lose clarity. Specifically, speech discrimination dysfunction, the ability to hear voices separately from background noise, may occur and involve: . -

Clinical Exome Sequencing for Genetic Identification of Rare Mendelian Disorders
Supplementary Online Content Lee H, Deignan JL, Dorrani N, Strom SP, Kantarci S, Quintero-Rivera F, et al. Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA. doi:10.1001/jama.2014.14604. eMethods 1. Sample acquisition and pre-test sample processing eMethods 2. Exome capture and sequencing eMethods 3. Sequence data analysis eMethods 4. Variant filtration and interpretation eMethods 5. Determination of variant pathogenicity eFigure 1. UCLA Clinical Exome Sequencing (CES) workflow eFigure 2. Variant filtration workflow starting with ~21K variants across the exome and comparing the mean number of variants observed from trio-CES versus proband-CES eFigure 3. Variant classification workflow for the variants found within the primary genelist (PGL) eTable 1. Metrics used to determine the adequate quality of the sequencing test for each sample eTable 2. List of molecular diagnoses made eTable 3. List of copy number variants (CNVs) and uniparental disomy (UPD) reported and confirmatory status eTable 4. Demographic summary of 814 cases eTable 5. Molecular Diagnosis Rate of Phenotypic Subgroups by Age Group for Other Clinical Exome Sequencing References © 2014 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 10/01/2021 This supplementary material has been provided by the authors to give readers additional information about their work. © 2014 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 10/01/2021 eMethods 1. Sample acquisition and pre-test sample processing. Once determined by the ordering physician that the patient's presentation is clinically appropriate for CES, patients were offered the test after a counseling session ("pre-test counseling") [eFigure 1]. -

Prenatalscreen® Standard Technical Report
About PrenatalScreen® Prenatal Test PrenatalScreen® Prenatal Test is a genetic test that analyses fetal DNA, obtained from CVS or amniotic fluid following an invasive prenatal diagnosis, to screen for monogenic disorders in the fetus. Using the latest technologies, including Next Generation Sequencing (NGS), PrenatalScreen® Prenatal Test screen 744 genes for mutations causing over 1.000 severe genetic disorders in the fetus. PrenatalScreen® Prenatal Test allows for a comprehensive care and enables patients to make more informed reproductive decisions. Offering PrenatalScreen® Prenatal Test to a patient during pregnancy allows her to gain more knowledge about the potential to pass along a condition to the fetus. Aim of the test PrenatalScreen® Prenatal Test analyses DNA extracted from fetal cells in the amniotic fluid, collected through amniocentesis, or in the chorionic villi through villocentesis (CVS). The aim of this diagnositc test is to assess severe genetic diseases in the fetus, including the most common diseases in the European population. Genes listed in Table 1 were selected according to the incidence in the population of the disease caused by mutations in such genes, the severity of the clinical phenotype at birth and the importance of the related pathogenetic picture, in accordance with the indications of the American College of Medical Genetics (ACMG)(Grody et al., Genet Med 2013:15:482–483). PrenatalScreen®: Indication for testing PrenatalScreen® Prenatal Test is intended for patients who meet any of the following criteria: • Personal/familial anamnesis of hereditary genetic diseases; • For expectant mothers wishing to reduce the risk of a genetic diseases in the fetus; • For natural or in vitro fertilization (IVF)-derived pregnancies: • For couples using heterologus IVF procedures (egg/sperm donors). -

A Mouse Forward Genetics Screen Identifies LISTERIN As an E3
A mouse forward genetics screen identifies INAUGURAL ARTICLE LISTERIN as an E3 ubiquitin ligase involved in neurodegeneration Jessie Chua,1, Nancy A. Hongb,2, Claudio A. Masudac,3, Brian V. Jenkinsa, Keats A. Nelmsd, Christopher C. Goodnowd, Richard J. Glynnec, Hua Wub,4, Eliezer Masliahe, Claudio A. P. Joazeiroc,5, and Steve A. Kaya,6,7 aDepartment of Biochemistry, Institute for Childhood and Neglected Diseases, The Scripps Research Institute, ICND216, 10550 North Torrey Pines Road, La Jolla, CA 90237; bPhenomix Corporation, 5871 Oberlin Drive, Suite 200, San Diego, CA 92121; cGenomics Institute of the Novartis Research Foundation, 10675 John Jay Hopkins Drive, San Diego, CA 92121; dPhenomix Australia, Pty., Ltd., Level 3 Building 117, Australian Phenomics Facility, Garran Road, Acton, ACT 2601, Australia; and eDepartment of Neurosciences, University of California San Diego, School of Medicine, La Jolla, CA 92093 This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected in 2008. Contributed by Steve A. Kay, December 19, 2008 (sent for review November 13, 2008) A mouse neurological mutant, lister, was identified through a ENU generated a rat model for human Usher syndrome type 1B genome-wide N-ethyl-N-nitrosourea (ENU) mutagenesis screen. (9). Further, ENU-induced mutation in dynein led to progressive Homozygous lister mice exhibit profound early-onset and progres- motor neuron degeneration in mice (10). Although no human sive neurological and motor dysfunction. lister encodes a RING disease has yet been mapped to dynein itself, mutation in the finger protein, LISTERIN, which functions as an E3 ubiquitin ligase dynein activator, dynactin, causes degeneration of lower motor in vitro.