Potential Application of Some Lamiaceae Species in the Management of Diabetes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Plants for Landscapes
Plus 10 Water-Saving Tips for your Garden your for Tips Water-Saving 10 Plus 5 Printed on recycled paper recycled on Printed © 2014 San Diego County Water Authority Water County Diego San 2014 © sdbgarden.org sdbgarden.org thegarden.org tted landscape that looks beautiful and saves water. saves and beautiful looks that landscape tted retrofi or new a for ideas get Landscapes ese gardens are excellent places to to places excellent are gardens Th ese Cajon. El in Garden Conservation Water the and Encinitas Many of the plants in this guide are labeled and on display at the San Diego Botanic Garden in in Garden Botanic Diego San the at display on and labeled are guide this in plants the of Many 0 WaterSmartSD.org Plants for for Plants Nifty agencies member 24 its and hese Nift y 50 plants have been selected because they are attractive, T oft en available in nurseries, non-invasive, easy to maintain, long- term performers, scaled for residential landscapes and, once estab- lished, drought-tolerant. In fact, these plants thrive in San Diego’s semi- arid climate and can help restore regional authenticity to your home. What’s exciting is that authentic also means sustainable. Plants native to Mediterranean climate zones love it here as much as you do. Th ey adapted over thousands of years, and the animal species that depend on them for food and habitat adapted, too. In fact, there are thousands of ground cov- ers, grasses, succulents, perennials, shrubs, vines and trees to choose from. For more information, go to WaterSmartSD.org. -

Crop Profile for Basil in New Jersey Ocimum Basilicum (L.) O
Crop Profile for Basil in New Jersey Ocimum basilicum (L.) O. tenuiflorum O. americanum Lamiales: Lamiaceae Prepared: 2008 General Production Information Yearly production: Approximately 450 acres % of crop for fresh market: 100% % of crop grown for retail market: 10 % % of crop grown for wholesale market: 90 % Production Regions The majority of basil production occurs in the southern New Jersey counties of Atlantic (100 acres), Cumberland (225 acres), and Gloucester (50 acres). There is some basil production in the central counties of Monmouth, Ocean, and Burlington, amounting to less than 50 acres. Soils in southern and much of central New Jersey are light, ranging from sand to sandy loam with some areas of silt loam. Basil production in the southern region extends from early April to October. In this region, basil is predominantly grown for the wholesale market, with plant stems generally cut or pulled, washed and bundled in 12-15 plant or stem bunches. Wholesale units are 15-count (bunch) crates. Some basil in this region is produced for the retail market and sold at farm stands or directly to restaurants. In this case, basil is generally sold by the bunch. In the northern counties of Hunterdon, Mercer, Morris, Warren, and Sussex, many growers produce basil, but on minimal acreage. Soils in the northern region are typically Piedmont (heavy silt loams) and Appalachian (shaley) soils. The field season begins in mid-May here, and continues until cold weather terminates production. In the north, basil is grown exclusively for retail at farm stands and at community-sponsored farmers markets. Typical growers in this region produce less than one half acre of basil. -

J. APIC. SCI. Vol. 59 No. 2 2015 DOI: 10.1515/JAS-2015-0028
DOI: 10.1515/JAS-2015-0028 J. APIC. SCI. Vol. 59 No. 2 2015J. APIC. SCI. Vol. 59 No. 2 2015 Original Article FLORAL PHENOLOGY, NECTAR SECRETION DYNAMICS, AND HONEY PRODUCTION POTENTIAL, OF TWO LAVENDER SPECIES (LAVANDULA DENTATA, AND L. PUBESCENS) IN SOUTHWESTERN SAUDI ARABIA Adgaba Nuru* Ahmad A. Al-Ghamdi Yilma T. Tena Awraris G. Shenkut Mohammad J. Ansari Anwer Al-Maktary Engineer Abdullah Bagshan Chair for Bee Research, Department of Plant Protec- tion, College of Food and Agricultural Science, King Saud University Riyadh 11451 Riyadh (P. Box 2460), Saudi Arabia *corresponding author: [email protected] Received 18 August 2015; accepted 07 October 2015 A b s t r a c t The aim of the current study was to determine the floral phenology, nectar secretion dynamics, and honey production potentials of two naturally growing lavender species (L. dentata and L. pubescens), in southwestern Saudi Arabia. In both species, flowering is continuous. This means that, when open flowers on a spike are shaded, new flowers emerge. Such a flowering pattern might be advantageous to the plant to minimise competition for pollinators and promote efficient resource allocation. The flowering periods of the two species overlap. Both species secreted increasing amounts of nectar from early morning to late afternoon. The mean maximum volumes of accumulated nectar from bagged flowers occurred at 15:00 for L. pubescens (0.50 ± 0.24 µL/flower) and at 18:00 for L. dentata (0.68 ± 0.19 µL/flower). The volume of the nectar that became available between two successive measurements (three-h intervals) varied from 0.04 µL/flower to 0.28 µL/flower for L. -
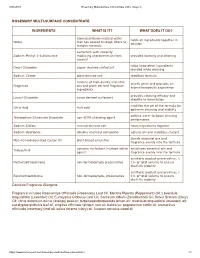
ROSEMARY MULTI-SURFACE CONCENTRATE INGREDIENTS WHAT IS IT? WHAT DOES IT DO? Contains Fragrance Allergens Fragrance Includes Rosm
9/24/2019 Rosemary Multi-Surface Concentrate | Mrs. Meyer's ROSEMARY MULTI-SURFACE CONCENTRATE INGREDIENTS WHAT IS IT? WHAT DOES IT DO? deionized/demineralized water holds all ingredients together in Water that has passed through filters to solution remove minerals surfactant with viscosity Sodium Methyl 2-Sulfolaurate modifying characteristics from provides foaming and cleaning coconut helps keep other ingredients Decyl Glucoside sugar- derived surfactant blended while cleaning Sodium Citrate plant-derived salt stabilizes formula mixture of high quality essential smells great and provides an Fragrance oils and plant-derived fragrance aromatherapeutic experience ingredients provides cleaning efficacy and Lauryl Glucoside sugar derived surfactant stability to formulation modifies the pH of the formula for Citric Acid fruit acid optimum cleaning and stability softens water to boost cleaning Tetrasodium Glutamate Diacetate non-EDTA chelating agent performance Sodium Sulfate mineral-derived salt holds ingredients together Sodium Hydroxide alkaline chemical compound adjusts pH and stabilizes chelant blends essential oils and PEG-40 Hydrogenated Castor Oil plant based emulsifier fragrance evenly into the formula nonionic surfactant (surface active solubilizes essential oils and Trideceth-9 agent) fragrance evenly into the formula synthetic product preservative, < Methylisothiazolinone non-formaldehyde preservative 1% of total volume to ensure shelf life stability synthetic product preservative, < Benzisothiazolinone non-formaldehyde, preservative -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

Review Article
Ramaiah Maddi et al / Int. J. Res. Ayurveda Pharm. 10 (3), 2019 Review Article www.ijrap.net A REVIEW ON OCIMUM SPECIES: OCIMUM AMERICANUM L., OCIMUM BASILICUM L., OCIMUM GRATISSIMUM L. AND OCIMUM TENUIFLORUM L. Ramaiah Maddi *, Prathi Amani, Singam Bhavitha, Tulluru Gayathri, Tummala Lohitha Department of Pharmacognosy, Hindu College of Pharmacy, Amravati Road, Guntur – 522002, A.P., India Received on: 25/02/19 Accepted on: 05/05/19 *Corresponding author E-mail: [email protected] DOI: 10.7897/2277-4343.100359 ABSTRACT Ocimum species (O.americanum, O.basilicum, O.gratissimum, and O.tenuiflorum) belongs to family Lamiaceae. It is also known as Tulsi. It is currently used as a traditional medicinal plant in India, Africa and other countries in the World. It is used in Ayurveda and in traditional Chinese medicine for treating different diseases and disorders like digestive system disorders such as stomach ache and diarrhea, kidney complaints, and infections, etc. Many researchers have investigated the anti-inflammatory potential of various Ocimum species and reported various activities like anti-viral, anti-bacterial, anti-hemolytic and also different phytoconstituents like essential oil, saponins, phenols, phlobatannins, and anthraquinones etc. Exploration of the chemical constituents of the plants and pharmacological activities may provide us the basis for developing new life-saving drugs hence this revieW may help the traditional healers, practitioners, researchers and students Who Were involved in the field of ethno pharmacology. Keywords: Ocimum species, Therapeutic uses, Biological activity, Phytoconstituents. INTRODUCTION varieties, as Well as several related species or hybrids Which are also called as basil. The type used commonly is typically called The name "basil" comes from Latin Word ‘Basilius’. -

Lamiaceae), with Emphasis on Taxonomic Implications
Biologia 67/5: 867—874, 2012 Section Botany DOI: 10.2478/s11756-012-0076-z Trichome micromorphology of the Chinese-Himalayan genus Colquhounia (Lamiaceae), with emphasis on taxonomic implications Guo-Xiong Hu1,3,TeodoraD.Balangcod2 & Chun-Lei Xiang1* 1Key Laboratory of Biodiversity and Biogeography, Kunming Institute of Botany, Chinese Academy of Sciences, 132 Lanhei Road, Heilongtan, Kunming 650201, Yunnan, P. R. China; e-mail: [email protected] 2Department of Biology, College of Science, University of the Philippines Baguio, 2600 Baguio City, Philippines 3Graduate School of Chinese Academy of Sciences, Beijing 100039,P.R.China Abstract: Trichome micromorphology of leaves and young stems of nine taxa (including four varieties) of Colquhounia were examined using light and scanning microscopy. Two basic types of trichomes were recognized: eglandular and glandular. Eglandular trichomes are subdivided into simple and branched trichomes. Based on the number of cells and trichome configuration, simple eglandular trichomes are further divided into four forms: unicellular, two-celled, three-celled and more than three-celled trichomes. Based on branching configuration, the branched eglandular trichomes can be separated into three forms: biramous, stellate and dendroid. Glandular trichomes can be divided into two subtypes: capitate and peltate glandular trichomes. Results from this study of morphological diversity of trichomes within Colquhounia lend insight into infrageneric classification and species relationships. Based on the presence of branched trichomes in C. elegans,thisspecies should be transferred from Colquhounia sect. Simplicipili to sect. Colquhounia. We provide a taxonomic key to species of Chinese Colquhounia based on trichome morphology and other important morphological traits. Key words: Colquhounia; glandular hairs; leaf anatomy; Lamioideae; Yunnan Introduction in spikes or capitula; equally 5–toothed calyces; and nutlets winged at apex (Li & Hedge 1994). -

Malacological Diversity on Four Lamiaceae in the Region of Tlemcen
Journal of Plant Sciences and Crop Protection Volume 1 | Issue 1 Review Article Open Access Malacological diversity on four Lamiaceae in the region of Tlemcen (Northwest of Algeria) Damerdji A* Department of Ecology and Environment, Faculty of SNV-STU, University of Tlemcen, Algeria *Corresponding author: Damerdji A, Department of Ecology and Environment, Faculty of SNV-STU, University of Tlemcen, 13000, Algeria, E-mail: [email protected] Citation: Damerdji A (2018) Malacological diversity on four Lamiaceae in the region of Tlemcen (North- west of Algeria). J Plant Sci Crop Protec 1(1): 106. doi: 10.15744/2639-3336.1.106 Received Date: March 07, 2018 Accepted Date: July 25, 2018 Published Date: July 27, 2018 Abstract The region of Tlemcen is located in the north-west Algeria. Tends arid climate leads to a degradation of vegetation in open formation, where are found the doum the diss and broom.... Other aromatic species are considered: rosemary, thyme, lavender and horehound. By their morphological and botanical four aromatic species belonging to the Labiatae family. We propose an approach to achieve diversity malacofauna identified on these Lamiaceae. These latters are certainly a nutritional source for this malacological fauna. For this, an inventory is made in different stations. Malacological wealth of thyme is estimated at 19, that the rosemary to 18, on 14 and lavender to last, that the horehound 7. It includes four families namely Milacidae the Sphincterochilidae the Helicidae and Subulinidae. Milacidae are present only in horehound and lavender stations. On the other hand, the Sphincterochilidae, namely Sphincterochila candidissima, is absent on horehound and lavander. -

Sharp's at Waterford Farm Your Neighborhood Farm Ask Us How To
Lemongrass – Essential for Thai Sharp’s at Waterford Herbs List cooking Farm Anise - Hyssop Lovage (Levistcum officinale) Farming in Howard County Basil Marjoram (Origanum majorana) since 1903 African Blue Amethyst Improved Purple Sweet Eleonora Zaatar, a hint of thyme, oregano & 4003 Jennings Chapel Rd. Elidia - Compact; container basil marjoram Brookeville, MD 20833 Genovese Golden - ornamental mostly Holy - Sacred Red and Green Tel: (410) 489-2572 Mint (Mentha sp.) Italian Large Leaf Chocolate Peppermint Lemon – Mrs. Burns www.sharpfarm.com Lemon Mint Mountain Mint Lettuce Leaf – Napoletano email: Peppermint Pineapple Mint Lime [email protected] Spearmint Sweet Thai Dark Opal Oregano (Origanum sp.) Red Rubin Greek Rutgers Devotion Zaatar ( a hint of thyme, oregano, & marjoram) Oreganum Syriaca) Borage: the herb of gladness Hot and Spicy - real tang, our favorite for adding to beans Catnip (Nepeta)- feline friends treat Parsley (Petroselinum crispum) Calendula, Neon Plain leaf (Italian or flat) Curly – double or triple Chamomile (German) Organic curled parsley (Bodegold) Italian Dark Green – Giant of Italy – huge leaves Your Neighborhood Chervil (Anthricus cerefolium) ‘crispum’ Vertissimo Farm Rosemary (Rosmarinus) Arp Chives (Allium) Hill Hardy Med Leaf (Purly) Ask Us How to Garden Salem Large leaf (staro) Sage (Salvia offincinalis) Helpful Hints: We pride ourselves Cilantro (Coriandrum sativium) Garden - Extrakta on knowing how to vegetable and herb Cruiser – more upright – great for Pineapple garden. Please ask if you need bunching – 50 days Savory Winter information on how to. Yields? Cutting Celery (Apium graveolens) Sorrel, French Spacing between plants? Staking? aka leaf celery When you plant, space your harvest Stevia (Stevia rebaudiana) by using varieties of different maturity Dill (Anethum graveolens): Nature’s natural sweetener dates. -

Checklist of the Vascular Alien Flora of Catalonia (Northeastern Iberian Peninsula, Spain) Pere Aymerich1 & Llorenç Sáez2,3
BOTANICAL CHECKLISTS Mediterranean Botany ISSNe 2603-9109 https://dx.doi.org/10.5209/mbot.63608 Checklist of the vascular alien flora of Catalonia (northeastern Iberian Peninsula, Spain) Pere Aymerich1 & Llorenç Sáez2,3 Received: 7 March 2019 / Accepted: 28 June 2019 / Published online: 7 November 2019 Abstract. This is an inventory of the vascular alien flora of Catalonia (northeastern Iberian Peninsula, Spain) updated to 2018, representing 1068 alien taxa in total. 554 (52.0%) out of them are casual and 514 (48.0%) are established. 87 taxa (8.1% of the total number and 16.8 % of those established) show an invasive behaviour. The geographic zone with more alien plants is the most anthropogenic maritime area. However, the differences among regions decrease when the degree of naturalization of taxa increases and the number of invaders is very similar in all sectors. Only 26.2% of the taxa are more or less abundant, while the rest are rare or they have vanished. The alien flora is represented by 115 families, 87 out of them include naturalised species. The most diverse genera are Opuntia (20 taxa), Amaranthus (18 taxa) and Solanum (15 taxa). Most of the alien plants have been introduced since the beginning of the twentieth century (70.7%), with a strong increase since 1970 (50.3% of the total number). Almost two thirds of alien taxa have their origin in Euro-Mediterranean area and America, while 24.6% come from other geographical areas. The taxa originated in cultivation represent 9.5%, whereas spontaneous hybrids only 1.2%. From the temporal point of view, the rate of Euro-Mediterranean taxa shows a progressive reduction parallel to an increase of those of other origins, which have reached 73.2% of introductions during the last 50 years. -

Medicinal Plants, Aromatic Herbs and Fragrance Plants in France
> Medicinal plants, aromatic herbs and fragrance plants in France: a small www.ihc2022.org but thriving sector with a strong traditional base and a dynamic research network Annabelle Bergoënd and Joséphine Piasentin In France, medicinal and aromatic plants paced growth. This production is very diverse small areas. Many producers reserve some (MAP) or herbal, medicinal and aromat- and dynamic, leaning on strong traditional areas as a side crop for their main produc- ic plants (HMAP) industries1 traditionally skills and benefiting from new techniques tion. Many of the plants are not referenced refer to culinary herbs, medicinal plants, and high value processing. With a growing in the European common agricultural pol- and plants cultivated for the fragrance and demand for natural products and unfolding icy (CAP) nomenclature. About 75% of the perfume industries. This agricultural sector opportunities for new markets, HMAP is fac- producers cultivate HMAPs to diversify their encompasses several hundred plant species, ing exciting prospects. production. The largest amount of land is as compared with field crops, such as cereals, occupied by fragrance plants, then medici- which may include single species grains. Characteristics of French HMAP nal plants. Aromatic herbs account for less The organic production for these crops is than 10% of the HMAP cultivated area. In significantly larger than traditional crops of Farm features and production are 2018, the estimated average farm was 4 ha global French agriculture. Wild harvest of geographically diverse for MAP production and 17 ha for perfume HMAP continues and is key for a success- The complexity of the production sector for plants. -

Vitro Determination of Sun Protection Factor of Ocimum Basilicum, Linn
International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 2, Suppl 4, 2010 Research Article FORMULATION AND IN VITRO DETERMINATION OF SUN PROTECTION FACTOR OF OCIMUM BASILICUM, LINN. LEAF OILS SUNSCREEN CREAM SHANTANU KALE, AMOL SONAWANE*, AMMAR ANSARI, PRASHANT GHOGE, ASHWINI WAJE Department of Pharmacognosy, MGV’s Pharmacy College, MumbaiAgra Highway, Panchvati. Nashik 422003 Received: 12 July 2010, Revised and Accepted: 23 August 2010 ABSTRACT The increasing awareness of the risk of skin cancer with the sun exposure requires that sunscreen products be approximately tested and labeled. Although there exists information on the possible photon‐induced reaction such as photoirritation and photosensitization produced by sunscreen creams containing synthetic photo‐protective chemicals. Effect of sunscreen cream depends upon sun protection factor (SPF) value and substantivity. In addition due to high cost and time consumption of in vivo SPF determination methodologies, in vitro SPF determination is gaining more importance. The aim of present study was to formulate a sunscreen cream containing essential oil of Ocimum basilicum Linn. as an active ingredient and evaluate experimental method for in vitro SPF determination. Keywords: Ocimum basilicum, UV Protection, Sun protection factor, Sunscreens INTRODUCTION Ocimum basilicum, Linn. (basil) belongs to the plant family Lamiaceae, sub‐family Nepetoideae, and the genus sensulato10. Plant Sunlight composed of various wavelengths ranging from commonly known as sweet or garden basil (Engl.), Munjariki (Sans.), ultraviolet light through infrared to visible light. Exposure to solar basilikumkraut (Ger.), Herbe de grand basilic (Fr.), Common radiation is recognized to have negative effects on the human skin. basil11,12. Lamiaceae plants are native through the subtropics, Among all, ultraviolet light is the most harmful to the skin and especially throughout the Mediterranean region.