For Predicting In-Hospital Mortality in Traumatic Brain Injury Patients
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prediction of Mortality Rate of Trauma Patients in Emergency Room at Cipto Mangunkusumo Hospital by Several Scoring Systems
Vol. 22, No. 4, November 2013 Prediction of mortality rate in trauma patients 227 Prediction of mortality rate of trauma patients in emergency room at Cipto Mangunkusumo Hospital by several scoring systems Pande M.W. Tirtayasa, Benny Philippi Department of Surgery, Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta, Indonesia Abstrak Latar belakang: Penanganan trauma dikenal sebagai salah satu tantangan utama pada pelayanan kesehatan saat ini. Sistem skor trauma yang mudah digunakan dapat memberi informasi pada dokter mengenai tingkat keparahan pasien dan membantu dalam pengambilan keputusan mengenai tatalaksana pasien berikutnya. Penelitian ini bertujuan untuk menentukan sistem skor trauma yang paling sesuai dengan membandingkan prediksi angka mortalitas menggunakan sistem skor: 1) triage-revised trauma score (T-RTS); 2)mekanisme, Glasgow coma scale (GCS), umur, dan tekanan arteri (MGAP); 3) dan GCS, umur, dan tekanan darah sistolik (GAP) pada pasien trauma di Instalasi Gawat Darurat (IGD) Rumah Sakit Cipto Mangunkusumo (RSCM). Metode: Penelitian dilakukan secara retrospektif menggunakan data status pasien dengan trauma yang datang ke ruang resusitasi IGD RSCM sepanjang tahun 2011. Sebanyak 185 pasien trauma ditangani di ruang resusitasi IGD RSCM. Kriteria inklusi adalah semua pasien trauma yang langsung datang atau dibawa ke ruang resusitasi IGD RSCM. Kriteria eksklusi adalah pasien rujukan dari rumah sakit lain dan semua pasien trauma anak (<18 tahun). Data dasar pasien dihitung berdasarkan masing-masing trauma skor. Luaran (pasien hidup atau meninggal) dicatat dalam 24 jam pertama sejak masuk rumah sakit. Hasil: Sebanyak 124 kasus dianalisis dengan rerata usia 32,4 tahun dan angka kesudahan meninggal sebanyak 23 (18,5%) kasus. Angka mortalitas kelompok risiko rendah pada system skor T-RTS, MGAP, dan GAP berturut-turut sebesar 5%, 1,3%, dan 1,4% (p = 1,000). -

Comparison of Revised Trauma Score, Injury Severity Score and Trauma and Injury Severity Score for Mortality Prediction in Elderly Trauma Patients
ORIGINAL ARTICLE Comparison of Revised Trauma Score, Injury Severity Score and Trauma and Injury Severity Score for mortality prediction in elderly trauma patients Shahrokh Yousefzadeh-Chabok, M.D.,1,2 Marieh Hosseinpour, M.D.,1 Leila Kouchakinejad-Eramsadati, M.D.,1 Fatemeh Ranjbar, M.D.,2 Reza Malekpouri, M.D.,1 Alireza Razzaghi, M.D.,1 Zahra Mohtasham-Amiri, M.D.1,3 1Guilan Road Trauma Research Center, Guilan University of Medical Sciences, Rasht-Iran 2Department of Neurosurgery, Guilan University of Medical Sciences, Rasht-Iran 3Department of Social and Preventive Medicine, Guilan University of Medical Sciences, Rasht-Iran ABSTRACT BACKGROUND: Trauma is the fifth leading cause of death in patients 65 years and older. This study is a comparison of results of Revised Trauma Score (RTS), Injury Severity Score (ISS), and Trauma and Injury Severity Score (TRISS) in prediction of mortality in cases of geriatric trauma. METHODS: This is a cross-sectional study of records of 352 elderly trauma patients who were admitted to Pour-Sina Hospital in Rasht between 2010 and 2011. Injury scoring systems were compared in terms of specificity, sensitivity, and cut-off points using re- ceiver operating characteristic curve of patient prognosis. RESULTS: Mean age of patients was 71.5 years. Most common mechanism of injury was traffic accident (53.7%). Of the total, 13.9% of patients died. Mean ISS was higher for patients who did not survive. Mean of TRISS and RTS scores in elderly survivors was higher than non-survivors and difference in all 3 scores was statistically significant (p<0.001). Best cut-off points for predicting mortality in elderly trauma patients in RTS, ISS, and TRISS systems were ≤6, ≥13.5, and ≤2, with sensitivity of 99%, 84%, and 95% and specificity of 62%, 62%, and 72%, respectively. -

Glasgow Coma Scale
CalculatedPOWERED BY Decisions Clinical Decision Support for Pediatric Emergency Medicine Practice Subscribers Glasgow Coma Scale Introduction: The Glasgow Coma Scale (GCS) estimates coma severity based on eye, verbal, and motor criteria. Click the thumbnail above to access the calculator. Points & Pearls • The Glasgow Coma Scale (GCS) allows provid- agreement between providers, training and ers in multiple settings and with varying levels education are available online from the GCS of training to communicate succinctly about a creators at www.glasgowcomascale.org. patient’s mental status. • Simpler scores that have been shown to per- • The GCS has been shown to have statistical form as well as the GCS in the prehospital and correlation with a broad array of adverse neuro- emergency department setting (for initial evalu- logic outcomes, including brain injury, need for ation); these are often contracted versions of the neurosurgery, and mortality. GCS itself. For example, the Simplified Motor • The GCS score has been incorporated into Score (SMS) uses the motor portion of the GCS numerous guidelines and assessment scores only. THE SMS and other contracted scores are (eg, Advanced Cardiac Life Support, Advanced less well studied than the GCS for outcomes Trauma Life Support, Acute Physiology and like long-term mortality, and the GCS has been Chronic Health Evaluation I-III, the Trauma and studied as trended over time, while the SMS has Injury Severity Score, and the World Federation not. of Neurologic Surgeons Subarachnoid Hemor- rhage Grading Scale) Critical Actions Although it has been adopted widely and in a Points to keep in mind: variety of settings, the GCS score is not intended • Correlation with outcome and severity is most for quantitative use. -

Revised Trauma Scoring System to Predict In-Hospital Mortality in the Emergency Department: Glasgow Coma Scale, Age, and Systolic Blood Pressure Score
Revised trauma scoring system to predict in-hospital mortality in the emergency department: Glasgow Coma Scale, Age, and Systolic Blood Pressure score The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Kondo, Yutaka, Toshikazu Abe, Kiyotaka Kohshi, Yasuharu Tokuda, E Francis Cook, and Ichiro Kukita. 2011. Revised trauma scoring system to predict in-hospital mortality in the emergency department: Glasgow coma scale, age, and systolic blood pressure score. Critical Care 15(4): R191. Published Version doi:10.1186/cc10348 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:10497285 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Kondo et al. Critical Care 2011, 15:R191 http://ccforum.com/content/15/4/R191 RESEARCH Open Access Revised trauma scoring system to predict in-hospital mortality in the emergency department: Glasgow Coma Scale, Age, and Systolic Blood Pressure score Yutaka Kondo1, Toshikazu Abe2*, Kiyotaka Kohshi3, Yasuharu Tokuda4, E Francis Cook5 and Ichiro Kukita1 Abstract Introduction: Our aim in this study was to assess whether the new Glasgow Coma Scale, Age, and Systolic Blood Pressure (GAP) scoring system, which is a modification of the Mechanism, Glasgow Coma Scale, Age, and Arterial Pressure (MGAP) scoring system, better predicts in-hospital mortality and can be applied more easily than previous trauma scores among trauma patients in the emergency department (ED). -

Practice Management Guidelines for the Prevention of Venous Thromboembolism in Trauma Patients: the EAST Practice Management Guidelines Work Group Frederick B
CLINICAL MANAGEMENT UPDATE The Journal of TRAUMA Injury, Infection, and Critical Care Practice Management Guidelines for the Prevention of Venous Thromboembolism in Trauma Patients: The EAST Practice Management Guidelines Work Group Frederick B. Rogers, MD, Mark D. Cipolle, MD, PhD, George Velmahos, MD, PhD, Grace Rozycki, MD, and Fred A. Luchette, MD J Trauma. 2002;53:142–164. he Eastern Association for the Surgery of Trauma ommendation means the recommendation is reasonably jus- (EAST) has taken a leadership role in the development tifiable, usually on the basis of a preponderance of Class II Tof evidenced-based practice guidelines for trauma.1 data. If there are not enough Class I data to support a Level These original guidelines were developed by interested I recommendation, they may be used to support a Level II trauma surgeons in 1997 for the EAST Web site (www.eas- recommendation. A Level III recommendation is generally t.org), where a brief summary of four guidelines was pub- only supported by Class III data. lished. A revised, complete, and significantly edited practice These practice guidelines address eight different areas of management guidelines for the prevention of venous throm- practice management as they relate to the prevention and boembolism in trauma patients is presented herein. diagnosis of venous thromboembolism in trauma patients. The step-by-step process of practice management guide- There are few Level I recommendations because there is a line development, as outlined by the Agency for Health Care paucity of Class I data in the area of trauma literature. We Policy and Research (AHCPR), has been used as the meth- believe it is important to highlight areas where future inves- odology for the development of these guidelines.2 Briefly, the tigation may bring about definitive Level I recommendations. -

Sequential Organ Failure Assessment (SOFA) Score
CalculatedPOWERED BY Decisions Clinical Decision Support for Emergency Medicine Practice Subscribers Sequential Organ Failure Assessment (SOFA) Score Introduction: The SOFA score predicts mortality risk for patients in the intensive care unit based on lab results and clinical data. Click the thumbnail above to access the calculator. Points & Pearls • The Sequential Organ Failure Assessment namically or to determine the success or failure of (SOFA) is a mortality prediction score that is an intervention in the ICU. based on the degree of dysfunction of 6 organ systems. • The score is calculated at admission and every Why to Use 24 hours until discharge, using the worst param- The SOFA score can be used to determine the eters measured during the prior 24 hours. level of organ dysfunction and mortality risk in • The scores can be used in several ways, including: ICU patients. » As individual scores for each organ to deter- mine the progression of organ dysfunction. » As a sum of scores on a single intensive care When to Use unit (ICU) day. • The SOFA can be used on all patients who » As a sum of the worst scores during the ICU are admitted to an ICU. stay. • It is not clear whether the SOFA is reliable for • The SOFA score stratifies mortality risk in ICU patients who were transferred from another patients without restricting the data used to ICU. admission values. Critical Actions Instructions Clinical prediction scores such as the SOFA and the Calculate the SOFA score using the worst value Acute Physiologic Assessment and Chronic Health for each variable in the preceding 24-hour Evaluation (APACHE II) can be measured on all period. -

Trauma Measure #5 Documentation of Glasgow Coma Score at Time of Initial Evaluation in TBI
Trauma Measure #5 Documentation of Glasgow Coma Score at Time of Initial Evaluation in TBI National Quality Strategy Domain: Communication and Care Coordination Measure Type (Process/Outcome): Process 2016 PQRS OPTIONS FOR INDIVIDUAL MEASURES: REGISTRY DESCRIPTION: Percentage of trauma activations for patients aged 18 years or older evaluated as part of a trauma activation or trauma consultation who have a traumatic intracranial hemorrhage on initial head CT for which a Glasgow Coma Score (GCS) at the time of initial evaluation is documented in the medical record within 1 hour of hospital arrival. INSTRUCTIONS: This measure is to be reported each time a patient is evaluated as part of a trauma activation or trauma consultation and is found to have a traumatic intracranial hemorrhage on initial head CT. It is anticipated that clinicians who are responsible for the initial trauma evaluation of TBI patients as specified in the denominator coding will submit this measure. Measure Reporting via Registry: Abbreviated Injury Scale (AIS) codes, patient demographics, and the medical record are used to identify patients who are included in the measure’s denominator. The listed numerator options are used to report the numerator of the measure. DENOMINATOR: All patients aged 18 years or older evaluated by an eligible professional as part of a trauma activation or trauma consultation who have a traumatic intracranial hemorrhage on initial head CT). Denominator Criteria: Patients aged 18 years or older AND Patients evaluated as part of a trauma activation -
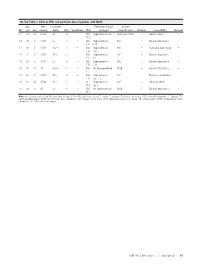
On-Line Table 1: Clinical, EEG, and Perfusion Data of Patients with NCSE Age Pre- Lat Signs/ Perfusion (Visual Seizure No
On-line Table 1: Clinical, EEG, and perfusion data of patients with NCSE Age Pre- Lat Signs/ Perfusion (visual Seizure No. (yr) Sex seizure Jerks GCS Ventilation EEG analysis) Classification Epilepsy Comorbidity Survival 1.1 59 M Coma R/Ϫ 6 ϩ SW Hyperperfusion Ins L Coma with NCSE – Cardiac arrest – FT L 1.2 72 F GTCS L/Ϫ 3 ϩ SW Hyperperfusion SSE ϩ Chronic infarction L ϩ PR PR 1.3 60 F GTCS No/Ϫ 3 ϩ SW Hyperperfusion SSE ϩ Traumatic brain injury ϩ FR FR 1.4 72 F GTCS R/ϩ 7 – SW Hyperperfusion SSE ϩ Chronic infarction L – TL TL 1.5 89 F GTCS L/Ϫ 8 – SW Hyperperfusion SSE ϩ Chronic infarction R – FR FR 1.6 67 F CP No/ϩ 11 – SW No Hyperperfusion CPSE ϩ Chronic infarction L ϩ FL 1.7 62 F GTCS R/Ϫ 5 ϩ SW Hyperperfusion SSE – Meningo-encephalitis – FR Ins,TL 1.8 67 M GTCS R/ϩ 5 ϩ SL Hyperperfusion SSE – Subacute SDH L ϩ FP L Ins L 1.9 63 F CP L/Ϫ 11 – SW No hyperperfusion CPSE ϩ Chronic infarction L ϩ TP L Note:—Lat indicates lateralizing; Pre, preceding: R, right; L, left; SW, spike-wave activity; F, frontal; T, temporal; P, parietal; Ins, insular; SDH, subdural hemorrhage; SL, slowing; EEG, electroencephalography; NCSE, nonconvulsive status epilepticus; GCS, Glasgow Coma Scale; GTCS, generalized tonic-clonic seizure; CP, complex-partial; CPSE, complex-partial status epilepticus; SSE, subtle status epilepticus. AJNR Am J Neuroradiol ͉͉www.ajnr.org E1 On-line Table 2: Clinical, EEG, and perfusion data of patients with a postictal state Age Pre- Lat Perfusion (visual No. -

SMJ-59-150.Pdf
Singapore Med J 2018; 59(3): 150-154 Original Article https://doi.org/10.11622/smedj.2017074 Management of computed tomography-detected pneumothorax in patients with blunt trauma: experience from a community-based hospital Ashraf F Hefny1,2, MD, Fathima T Kunhivalappil3, MD, Nikolay Matev3, MD, Norman A Avila4, BSN, Masoud O Bashir1, MD, Fikri M Abu-Zidan2, MD, PhD INTRODUCTION Diagnoses of pneumothorax, especially occult pneumothorax, have increased as the use of computed tomography (CT) for imaging trauma patients becomes near-routine. However, the need for chest tube insertion remains controversial. We aimed to study the management of pneumothorax detected on CT among patients with blunt trauma, including the decision for tube thoracostomy, in a community-based hospital. METHODS Chest CT scans of patients with blunt trauma treated at Al Rahba Hospital, Abu Dhabi, United Arab Emirates, from October 2010 to October 2014 were retrospectively studied. Variables studied included demography, mechanism of injury, endotracheal intubation, pneumothorax volume, chest tube insertion, Injury Severity Score, hospital length of stay and mortality. RESULTS CT was performed in 703 patients with blunt trauma. Overall, pneumothorax was detected on CT for 74 (10.5%) patients. Among the 65 patients for whom pneumothorax was detected before chest tube insertion, 25 (38.5%) needed chest tube insertion, while 40 (61.5%) did not. Backward stepwise likelihood regression showed that independent factors that significantly predicted chest tube insertion were endotracheal intubation (p = 0.01), non-United Arab Emirates nationality (p = 0.01) and pneumothorax volume (p = 0.03). The receiver operating characteristic curve showed that the best pneumothorax volume that predicted chest tube insertion was 30 mL. -

The Inclusion of Delirium in Version 2 of the National Early
ORIGINAL ORIGINAL RESEARCH RESEARCH ClinicalClinical Medicine Medicine 2019 2017 Vol Vol 19, 17, No No 2: 6:104–8 104–8 CORE Metadata, citation and similar papers at core.ac.uk Provided by Bradford Scholars The inclusion of delirium in version 2 of the National Early Warning Score will substantially increase the alerts for escalating levels of care: findings from a retrospective database study of emergency medical admissions in two hospitals Authors: M o h a m m e d A M o h a m m e d , A M u h a m m a d F a i s a l , B D o n a l d R i c h a r d s o n ,C A n d y S c a l l y , D R o b i n H o w e s , E K e v i n B e a t s o n , F S a l l y I r w i n G a n d K e v i n S p e e d H B a c k g r o u n d emergency medical patients. Rigorous evaluation of NEWS2 The National Early Warning Score (NEWS) is being replaced is required before widespread implementation because the with NEWS2 which adds 3 points for new confusion or delirium. extent to which staff can cope with this increase without We estimated the impact of adding delirium on the number of adverse consequences remains unknown. medium/high level alerts that are triggers to escalate care. -

The Impact of Early Warning Scoring Systems
General care Insights The impact of Early Warning Scoring systems: Better health outcomes, lower cost of care, and improved staff, patient and family experience Unmet needs and solutions: Response to the burden of patient deterioration Sizing the problem of patient deterioration Patient deterioration is a significant clinical and financial Patient deterioration may also result in a significant direct burden for patients, providers, and healthcare systems.2 economic burden for providers. Patients experiencing (Figure 1) Clinicians are increasingly treating older, sicker adverse events are associated with higher direct populations at risk for complications and in-hospital healthcare costs.9 adverse events.3 As the volume of hospitalizations for these patients continues to rise, providers are challenged A review of the literature highlights this trend, especially to manage more acute, resource intensive populations in among potentially preventable conditions: a resource-constrained environment.3 • Surgical complications were associated with a nearly $20K increase in hospital costs and a pronounced Compounded with a scarcity of beds in higher acuity decrease in contribution margin10 care areas, patient status may be underestimated, and • Median costs associated with sepsis are markedly patients may be admitted or transferred to lower acuity higher than in non-septic patients per case ($10K care areas while still at risk for deterioration and resulting United Kingdom, $23K Germany)11 serious adverse events. • $3,580 average cost per day of hospitalization for This scenario may be attributable to a relative lack of recipients of cardio pulmonary resuscitation12 resources in lower acuity settings, a significant decrease in nurse to patient ratios, and/or a lack of care coordination. -

Injury Severity Scoring
INJURY SEVERITY SCORING Injury Severity Scoring is a process by which complex and variable patient data is reduced to a single number. This value is intended to accurately represent the patient's degree of critical illness. In truth, achieving this degree of accuracy is unrealistic and information is always lost in the process of such scoring. As a result, despite a myriad of scoring systems having been proposed, all such scores have both advantages and disadvantages. Part of the reason for such inaccuracy is the inherent anatomic and physiologic differences that exist between patients. As a result, in order to accurately estimate patient outcome, we need to be able to accurately quantify the patient's anatomic injury, physiologic injury, and any pre-existing medical problems which negatively impact on the patient's physiologic reserve and ability to respond to the stress of the injuries sustained. Outcome = Anatomic Injury + Physiologic Injury + Patient Reserve GLASGOW COMA SCORE The Glasgow Coma Score (GCS) is scored between 3 and 15, 3 being the worst, and 15 the best. It is composed of three parameters : Best Eye Response, Best Verbal Response, Best Motor Response, as given below: Best Eye Response (4) Best Verbal Response (5) Best Motor Response (6) 1. No eye opening 1. No verbal response 1. No motor response 2. Eye opening to pain 2. Incomprehensible sounds 2. Extension to pain 3. Eye opening to verbal 3. Inappropriate words 3. Flexion to pain command 4. Confused 4. Withdrawal from pain 4. Eyes open spontaneously 5. Orientated 5. Localising pain 6. Obeys Commands Note that the phrase 'GCS of 11' is essentially meaningless, and it is important to break the figure down into its components, such as E3 V3 M5 = GCS 11.