Characterization Analysis of Raw and Pyrolyzed Plane Tree Seed (Platanus Orientalis L.) Samples for Its Application in Carbon Capture and Storage (CCS) Technology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Platanus Orientalis L.) in Kashmir Valley
An early archaeological evidence of Plane tree (Platanus orientalis L.) In Kashmir Valley Farooq A Lone, G. M. Buth & Maqsooda Khan Lone, FarooC] A., Buth, G. M. & Khan, Maqsooda 1990. An early archaeological evidence of Plane tree (Platanus orientalis L.) in Kashmir Valley. Palaeobotanist 37(3) : 389-391. An evidence of Platanus orientalis L. (Chinar) in charcoal samples from an archaeological site at Semthan in Kashmir Valley has brought to light the earliest record of this European tree dating back to early historic period from 500 to 1,000 A.D. The archaeological significance pertaining to its much earlier introduction in Kashmir Valley has been discussed. Key-words-Archaeobotany, Platanus, Exotic tree, Kashmir Valley (India). Farooq A. Lone, G. M. Buth & Maqsooda Khan, Department of Botany, University of Kashmir, Srinagar 190 006, India. ~T ~ q(I"I~'" ~ ~ d 'it ~ ~ (~ :u1~iVli\1tl ~o) .. ~ ~ ~ ~ l:!;o FIR, 'Jffi""f i(O l:!;cl ~ ~ ~ ~ ~ 'i<Ii1lf~qq> ~'lR ~ ~o.a q>T'l!O-~ ~ ~ ~ ai\fi:~·~f'<16 ~o (~) ll'ltT -q' -q' <f; <tit ~ ~ ~ ~~ ~ ~ ~ ~ ~ ~ <f; tl W Iflll"IT <f; 3lTW< '1< <f; lfUl' <m'1ft'< mit -q' W <tit mft tl W ~ ~ ~ ~ ~ ~ me--Q'lf -q' <m'1ft'< ll'ltT -q' W <f; aft"< 3!fuq> '1< q<Ii1If'<oCl q> <tit 'ITt t I THE study of wood remains recovered from the DESCRIPTION archaeological excavations has revealed not only the type of vegetation growing and the way of PI. 1, figs 1-4 exploitation of forest trees by toe ancient inhabitants Wood diffuse to semi-ring-porous. Growth rings but also prOVided important information on the marked by a band of fibres. -

Platanus Orientalis (Asian Planetree) This Plant Has Spreading Tall Branches That Are Covered in Flaking Grey and Tan Bark
Platanus orientalis (Asian Planetree) This plant has spreading tall branches that are covered in flaking grey and tan bark. It is also called as the Asian planetree and it is a native of southeastern Europe/Asia. Decidous and tall, this tree historically became famous for its characteristic of a shady tree in the Middle East. Unfolding to a large, narrowed semi-glossy blades, the leaves emerge in spring. They have a color of bright, pale green and many-teethed lobes that number between three and five. First the leaves emerge and then the flower clusters appear. The flowers are monoecious colored salmon and appear in clusters across branches. It has a large teardrop shape with vertically held flattened branchlets. Mostly the young plants can be seen as shrubs but it can be pruned into a small tree by eliminating the small branches. Landscape Information French Name: Platane d'Orient ﺩﻟﺐ ﻣﺸﺮﻗﻲ :Arabic Name Pronounciation: PLAT-uh-nus or-ee-en-TAY- liss Plant Type: Tree Origin: Europe/Southern Asia Heat Zones: 5, 6, 7, 8, 9 Hardiness Zones: 3, 4, 5, 6, 7, 8 Uses: Specimen, Border Plant, Shade, Street Size/Shape Growth Rate: Fast Tree Shape: Pyramidal, Round Canopy Symmetry: Symmetrical Canopy Density: Medium Canopy Texture: Coarse Height at Maturity: 8 to 15 m, 15 to 23 m, Over 23 Spread at Maturity: 8 to 10 meters, 10 to 15 meters, Over 15 meters Time to Ultimate Height: 20 to 50 Years Plant Image Platanus orientalis (Asian Planetree) Botanical Description Foliage Leaf Arrangement: Alternate Leaf Venation: Palmate Leaf Persistance: Deciduous -

Platanus Orientalis Oriental Planetree1 Edward F
Fact Sheet ST-485 October 1994 Platanus orientalis Oriental Planetree1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION This deciduous tree is one of the parents of the popular London Planetree (Platanus x acerifolia) and has more deeply lobed leaves than its offspring (Fig. 1). Leaves are almost maple-like. Capable of reaching 80 feet in height, the Oriental Planetree has very strong branches and is quite useful as a shade tree. The wood is so tough, dense and hard it is often used for butcher’s blocks and furniture. The springtime flowers are followed by fruits which are found on stalks in groups of three to 6. The attractive bark is cream colored and flaky and very striking in the winter. GENERAL INFORMATION Figure 1. Mature Oriental Planetree. Scientific name: Platanus orientalis Pronunciation: PLAT-uh-nus or-ee-en-TAY-liss Common name(s): Oriental Planetree Crown density: moderate Family: Platanaceae Growth rate: fast USDA hardiness zones: 7 through 9A (Fig. 2) Texture: coarse Origin: not native to North America Uses: shade tree; no proven urban tolerance Foliage Availability: grown in small quantities by a small number of nurseries Leaf arrangement: alternate (Fig. 3) Leaf type: simple DESCRIPTION Leaf margin: lobed; incised Leaf shape: ovate; star-shaped Height: 70 to 80 feet Leaf venation: palmate Spread: 50 to 70 feet Leaf type and persistence: deciduous Crown uniformity: symmetrical canopy with a Leaf blade length: 4 to 8 inches regular (or smooth) outline, and individuals have more Leaf color: green or less identical crown forms Fall color: yellow Crown shape: round; pyramidal 1. -
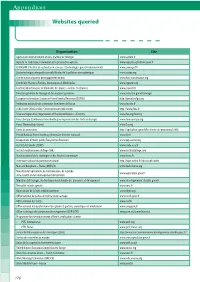
Appendices Websites Queried
Appendices Websites queried Organization Site Agence de l’environnement et de la maîtrise de l’énergie www.ademe.fr Agreste: la statistique, l’évaluation et la prospective agricole www.agreste.agriculture.gouv.fr CEMAGREF (l’Institut de recherche en sciences et technologies pour l’environnement) www.cemagref.fr Centre technique interprofessionnel d’études de la pollution atmosphérique www.citepa.org Comité national pour le développement du bois www.bois-construction.org Comité des Plantes à Parfum, Aromatiques et Médicinales www.cpparm.org Confédération française de l’industrie des papiers, cartons et celluloses www.copacel.fr Direction générale de l’énergie et des matières premières www.industrie.gouv.fr/energie European Information System on Forest Genetic Resources (EUFGIS) http://portal.eufgis.org Fédération nationale des communes forestières de France www.fncofor.fr FCBA (Forêt Cellulose Bois-Construction Ameublement) http://www.fcba.fr Food and Agriculture Organization of the United Nations – Forestry www.fao.org/forestry Forest Europe (Conférence ministérielle pour la protection des forêts en Europe) www.foresteurope.org Forest Stewardship Council www.fsc.org Forêts de protection http://agriculture.gouv.fr/les-forets-de-protection,10806 French National Forest Inventory (Inventaire forestier national) www.ifn.fr Groupement d’intérêt public Écosystèmes Forestiers www.gip-ecofor.org Institut de l’abeille (ITSAP) www.cnda.asso.fr Institut méditerranéen du liège (IML) www.institutduliege.com Institut national de la statistique et des -

Trees of the Bible: a Cultural History by Dr
Pub. No. 43 October 2016 Trees of the Bible: A Cultural History by Dr. Kim D. Coder, Professor of Tree Biology & Health Care Warnell School of Forestry & Natural Resources, University of Georgia In your backyard, within parks, hidden in forests, and along roadways, are local trees related to those mentioned in the Bible. More than 36 trees are mentioned throughout the Old and New Testa- ments. Some of these trees have relatives living here in the Southeastern United States. There is significant disagreement across time about identification of tree species mentioned in the Bible. In multiple translations from many places using different sources, some authors have reached different conclusions about what specific trees were mentioned in the Bible. The Bible is not a botanical treatise, and so modern tree identification accuracy is not relevant. Ancient Land The land of the Bible 3,000 years ago was starting to experience human development pressure, soil erosion and over-grazing which would lead to the landscapes of the modern Middle East. Natural resources present in great supply of the distant past have now dwindled to isolated remnants, included many tree species. Trees mentioned in the Bible can still be found in the wild places of the Middle East today. The Middle East area of the Bible can be generally described as historic Palestine. The area of Palestine today is made of several nations and many peoples. Historic Palestine was at the Eastern end of the Mediterranean Sea where Africa, Asia, and the Mediterranean Basin meet. This area has been cross roads for plant and plant product trade over millennium. -

Short Topic Assessment on Forests and Article 17 Related Data
European Environment Agency European Topic Centre on Biological Diversity Technical paper N° 5/2015 Short topic assessment on Forests and Article 17 related data. Analysis of Articles 12 and 17 reporting data from 2007-2012 for woodland and forest ecosystems Ben Delbaere, Celia García Feced and Sophie Condé 22/07/2015 The European Topic Centre on Biological Diversity (ETC/BD) is a consortium of twelve organisations under a Framework Partnership Agreement with the European Environment Agency for the period 2014-2018 ALTERRA AOPK-CR ECNC Ecologic ILE-SAS ISPRA JNCC MNHN SC-NAT SLU Space4environment UBA Authors’ affiliation: Ben Delbaere, European Centre for Nature Conservation (NL) Celia García Feced, Muséum national d’Histoire naturelle (FR) Sophie Condé, Muséum national d’Histoire naturelle (FR) EEA project manager: Anne-Marie Bastrup-Birk, European Environment Agency (DK) ETC/BD production support: Muriel Vincent, Muséum national d’Histoire naturelle (FR) Context: The Topic Centre has prepared this Technical paper in collaboration with the European Environment Agency (EEA) under its 2015 work programmes as a contribution to the EEA’s work on Biodiversity and ecosystem assessments and networks contributing to European and global processes. Citation: Please cite this report as Delbaere, B., García Feced, C. and Condé, S., 2015. Short topic assessment on Forests and Article 17 related data. ETC/BD report to the EEA. Disclaimer: This European Topic Centre on Biological Diversity (ETC/BD) Technical Paper has not been subject to a European Environment Agency (EEA) member country review. The content of this publication does not necessarily reflect the official opinions of the EEA. -

Platanus Orientalis, a Divine Gift for Greece
Platanus orientalis, a divine gift for Greece Dr Stephanos Diamandis Senior Forest Pathologist at the National Agricultural Research Foundation, Forest Research Institute All over Greece, from the very north to the extreme south, on the mainland and islands alike, in villages or country towns, one finds magnificent, age-old plane trees draped gracefully over churchyards or main squares. In the squares, where all the cafés and tavernas arrange their tables on hot summer days, these noble trees provide cool and pleasant shade. Plane trees also grow naturally along the banks of any river or stream, pond, lake, or spring, domi- nating the landscape. Indeed, it seems that God offered this tree as a gift to Greek nature! Platanus orientalis L., the Oriental plane, is endemic in Crete and other south- ern islands, but has since ancient times also been transplanted all over the mainland of Greece. Its natural distribution extends from the southern Balkans, Crete, the mountains of S.Turkey, W.Syria, N.Iraq and Iran, eastwards to Kashmir, where it is known as the ‘chenar’. Throughout its range, it is always linked to the existence of water, and appreciated for its deep shade and beautiful autumn colour. The Ancient Greeks considered this tree a divine gift; Homer, in the Iliad, mentions a plane tree in Avlis where the Greeks gathered their fleet to campaign against Troy, while Hippocrates taught his students in the shade of a huge plane tree on the island of Kos. Amazingly, a stump sprout of that original tree, which lived over 2500 years ago, still exists in one of the main squares in Kos Town. -

Seeds and Plants Imported
Issued March 30, U. S. DEPARTMENT OF AGRICULTURE. BUREAU OF PLANT INDUSTRY. WILLIAM A. TAYLOR, Chitf of Bureau. INVENTORY OF SEEDS AND PLANTS IMPORTED BY THE OFFICE OF FOREIGN SEED AND PLANT INTRODUCTION DURING THE PERIOD FROM APRIL 1 TO JUKE 30, 1915. (No. 43; Nos. 40389 TO 40895.) WASHINGTON: GOVERNMENT PRINTING CPPIOE. Issued March 30,1918. U. S. DEPARTMENT OF AGRICULTURE. BUREAU OF PLANT INDUSTRY. WILLIAM A. TAYLOR, Chief of Bureau. INVENTORY OF SEEDS AND PUNTS IMPORTED OFFICE OF FOREIGN SEED AND PLANT INTRODUCTION DURING THE PERIOD FROM APRIL 1 TO JUNE 30, 1915. (No. 43; Nos. 40389 TO 40895. ) WASHINGTON: GOVERNMENT PRINTING CFFIOE, 191& BUREAU OF PLANT INDUSTRY. Chief of Bureau, WILLIAM A. TAYLOR. Associate Chief of Bureau, KARL F. KBLLERMAN. Officer in Charge of Publications, J. E. ROCKWELL. Chief Cleric, JAMBS E. JONES. FOREIGN SEED AND PLANT INTRODUCTION. SCIENTIFIC STAFF. David Fairchild, Agricultural Explorer in Charge. P. H. Dorsett, Plant Introducer, in Charge of Plant Introduction Field Stations. B. T. Galloway, Plant Pathologist, in Charge of Plant Protection and Plant Propagation. Peter Bisset, Plant Introducer, in Charge of Foreign Plant Distribution. Frank N. Meyer, Wilson Popenoe, and F. C. Reimer, Agricultural Explorers. H. C. Skeels, S. C. Stuntz, and R. A. Young, Botanical Assistants. D. A. Bisset, R. N. Jones, P. G. Russell, and Glen P. Van Eseltine, Assistants. Robert L. Beagles, Superintendent, Plant Introduction Field Station, Chico, Cal. E. O. Orpet, Assistant in Plant Introduction, Chico, Cal. Edward Simmonds, Superintendent, Plant Introduction Field Station, Miami, Fla. John M. Rankin, Superintendent, Yarrow Plant Introduction Field Station, Rockville, Md. -

BVOC Emissions, Photosynthetic Characteristics and Changes in Chloroplast Ultrastructure of Platanus Orientalis L
Environmental Pollution 157 (2009) 2629–2637 Contents lists available at ScienceDirect Environmental Pollution journal homepage: www.elsevier.com/locate/envpol BVOC emissions, photosynthetic characteristics and changes in chloroplast ultrastructure of Platanus orientalis L. exposed to elevated CO2 and high temperature Violeta Velikova a,*, Tsonko Tsonev a, Csengele Barta b, Mauro Centritto c, Dimitrina Koleva d, Miroslava Stefanova d, Mira Busheva e, Francesco Loreto c a Bulgarian Academy of Sciences, Institute of Plant Physiology, Acad. G. Bonchev, Bl. 21, 1113 Sofia, Bulgaria b US Department of Agriculture, Agricultural Research Center, Arid-Land Agricultural Research Center (USDA, ARS) Maricopa, AZ, USA c Consiglio Nazionale delle Ricerche, Istituto di Biologia Agroambientale e Forestale, 00016 Monterotondo Scalo (RM), Italy d Sofia University, Faculty of Biology, 1000 Sofia, Bulgaria e Bulgarian Academy of Sciences, Institute of Biophysics, 1113 Sofia, Bulgaria Isoprene biosynthesis has a protective role on the photosynthetic machinery of Platanus plants exposed to changing environment (i.e., interaction between rising [CO2] and heat wave). article info abstract Article history: To investigate the interactive effects of increasing [CO2] and heat wave occurrence on isoprene (IE) and Received 27 January 2009 methanol (ME) emissions, Platanus orientalis was grown for one month in ambient (380 mmol molÀ1)or Received in revised form À1 elevated (800 mmol mol ) [CO2] and exposed to high temperature (HT) (38 C/4 h). In pre-existing 27 April 2009 leaves, IE emissions were always higher but ME emissions lower as compared to newly-emerged leaves. Accepted 3 May 2009 They were both stimulated by HT. Elevated [CO2] significantly reduced IE in both leaf types, whereas it increased ME in newly-emerged leaves only. -

Vision-Based Decision-Making Methodology for Riparian Forest Restoration and Flood Protection Using Nature-Based Solutions
sustainability Article Vision-Based Decision-Making Methodology for Riparian Forest Restoration and Flood Protection Using Nature-Based Solutions Maria A. Lilli 1,*, Sofia D. Nerantzaki 1, Christos Riziotis 1, Manolis Kotronakis 1, Dionissis Efstathiou 1, Dimitris Kontakos 2,3, Petros Lymberakis 2, Manolis Avramakis 2, Antonis Tsakirakis 2, Konstantinos Protopapadakis 1 and Nikolaos P. Nikolaidis 1 1 Department of Environmental Engineering, Technical University of Crete, 73100 Chania, Greece; [email protected] (S.D.N.); [email protected] (C.R.); [email protected] (M.K.); [email protected] (D.E.); [email protected] (K.P.); [email protected] (N.P.N.) 2 Natural History Museum of Crete, School of Science and Engineering, University of Crete, 71409 Heraklion, Greece; [email protected] (D.K.); [email protected] (P.L.); [email protected] (M.A.); [email protected] (A.T.) 3 Management Body of Samaria National Park-Western Crete, Old National Road Chania-Kissamos, Kato Daratso, 73100 Chania, Greece * Correspondence: [email protected]; Tel.: +30-2821037784 Received: 26 March 2020; Accepted: 16 April 2020; Published: 18 April 2020 Abstract: Nature-based solutions (NBS) are actions that use natural processes in a resource efficient manner to solve societal challenges. The lack of supportive legislature, and financial, communication and social barriers complicate the process of NBS implementation. It is an urgent need to develop approaches to design and implement NBS that would act as drivers to overcome potential barriers and enhance the social acceptability of the project. The vision-based decision-making methodology and participatory process created in this study has been carried out in the Koiliaris Critical Zone Observatory in Crete to design erosion and flood protection NBS and restore the riparian forest. -

Platanus × Acerifolia Platanus × Acerifolia, the London Plane, London Planetree, Or Hybrid Plane, Is a Tree in the Genus Platanus
Platanus × acerifolia Platanus × acerifolia, the London plane, London planetree, or hybrid plane, is a tree in the genus Platanus. It is usually thought to be a hybrid of Platanus orientalis (oriental plane) and Platanus occidentalis (American sycamore). Some authorities think that it may be a cultivar of P. orientalis. Description The London plane is a large deciduous tree growing 20–30 m (66–98 ft), exceptionally over 40 m (131 ft) tall, with a trunk up to 3 m (10 ft) or more in circumference. The bark is usually pale grey- green, smooth and exfoliating, or buff-brown and not exfoliating. The leaves are thick and stiff- textured, broad, palmately lobed, superficially maple-like, the leaf blade 10–20 cm (4–8 in) long and 12–25 cm (5–10 in) broad, with a petiole 3–10 cm (1–4 in) long. The young leaves in spring are coated with minute, fine, stiff hairs at first, but these wear off and by late summer the leaves are hairless or nearly so. The flowers are borne in one to three (most often two) dense spherical inflorescences on a pendulous stem, with male and female flowers on separate stems. The fruit matures in about 6 months, to 2–3 cm diameter, and comprises a dense spherical cluster of achenes with numerous stiff hairs which aid wind dispersal; the cluster breaks up slowly over the winter to release the numerous 2–3 mm seeds. The London Plane is one of the most efficient trees in removing small particulate pollutants in urban areas. It shares many visual similarities with Platanus occidentalis (American sycamore), of which it is derived; however, the two species are relatively easy to distinguish, considering the London plane is almost exclusively planted in urban habitats, while P. -

Distribution Pattern and Phytosociological Study of Trees Associated with Agroforestry in Poonch District of Jammu and Kashmir, India
Journal of Bioresource Management Volume 8 Issue 1 Article 8 Distribution Pattern and Phytosociological study of Trees Associated with Agroforestry in Poonch District of Jammu and Kashmir, India Javed Manzoor Govt Degree College Mendhar, Jammu and Kashmir, [email protected] Mohd Junaid Jazib Govt Postgraduate College Rajouri, Jammu and Kashmir, [email protected] Follow this and additional works at: https://corescholar.libraries.wright.edu/jbm Part of the Agriculture Commons, Biodiversity Commons, and the Forest Sciences Commons Recommended Citation Manzoor, J., & Jazib, M. J. (2021). Distribution Pattern and Phytosociological study of Trees Associated with Agroforestry in Poonch District of Jammu and Kashmir, India, Journal of Bioresource Management, 8 (1). DOI: https://doi.org/10.35691/JBM.1202.0167 ISSN: 2309-3854 online (Received: Oct 8, 2020; Accepted: Dec 19, 2020; Published: Mar 15, 2021) This Article is brought to you for free and open access by CORE Scholar. It has been accepted for inclusion in Journal of Bioresource Management by an authorized editor of CORE Scholar. For more information, please contact [email protected]. Distribution Pattern and Phytosociological study of Trees Associated with Agroforestry in Poonch District of Jammu and Kashmir, India © Copyrights of all the papers published in Journal of Bioresource Management are with its publisher, Center for Bioresource Research (CBR) Islamabad, Pakistan. This permits anyone to copy, redistribute, remix, transmit and adapt the work for non-commercial purposes provided the original work and source is appropriately cited. Journal of Bioresource Management does not grant you any other rights in relation to this website or the material on this website.