Age Related Memory Loss Arml Program and Cognitive Aging And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Web Site Links Gators to Gators Around the World
Your campus news source In Focus Note This Produced by the University Relations Office Benefits open enrollment for faculty, staff and students Web site links Gators to Gators around the world under way next week of the University of Florida Soon, accessing the Gator Nation will “nuggets” will appear from within the mercials, listen to new radio spots, and chat UF employees who wish to make be a whole lot easier. video, from behind a photograph and in with alumni and friends through a message changes to their pretax benefits may news.ufl.edu/insideuf Beginning Sept. 16, University of Florida other unexpected ways. board. do so during open enrollment Sept. alumni, students and friends of the universi- For example, Hice said, goGatorNation. “The interactive part is the ability to September 12, 2006 19 through Oct. 18. Changes made to ty will be able to upload their own personal com will feature facts about the university upload your own Go Gator commercial. elections at this time will be effective video and photos related to their experiences such as the $518 million in research grants It’s the next step of what we started a year Jan. 1. The Blog Top of Page at UF via goGatorNation.com, a new Web awarded to UF in 2006, as well as infor- ago,” Hice said, referring to the Gator site that aims to highlight interesting facts mation about former Nation campaign that Representatives from a number about the university and its alumni in a fun students such as the two included TV and radio of vendors will be on hand to answer Involve yourself and engaging way. -

ADDRESS1 BLDG BUILDING NAME ABBREV 100 NW 20TH ST 0153 Earl and Christy Powell Hall ODAA 100 NW 20Th ST 0253 University Foundation Annex UFFX 1002 W
ADDRESS1 BLDG BUILDING_NAME ABBREV 100 NW 20TH ST 0153 Earl and Christy Powell Hall ODAA 100 NW 20th ST 0253 University Foundation Annex UFFX 1002 W. University Avenue 3408 Tau Kappa Epsilon TKE 1006 CENTER DR 0723 Chemical Engineering CHE 1006 CENTER DR 0869 Chemical Engineering Digester CEDG 1026 MAGNOLIA DR 0705 Facilities Services Central Stores FSCS 1030 CENTER DR 0958 Chemical Engineering Student Center CESC 1037 MAGNOLIA DR 0706 Facilities Services Motor Pool FSMP 1041 CENTER DR 0070 Nanoscale Research Facility NANO 1048 GALE LEMERAND DR 0579 Reclaimed Water Storage Facility 105 GALE LEMERAND DR 0160 Heritage Hall HER 105 NW 16th ST 0105 The 105 Classroom Building CBD 1062 MUSEUM RD 0508 NS Field Station NSFS 1063 ELMORE DR 0437 Fiber Hut Elmore 1064 CENTER DR 0033 Engineering NEB 110 FLETCHER DR 0135 Albert A. Murphree Hall 1101 MUSEUM DR 0982 Baughman Support Building BAU1 1101 MUSEUM DR 0983 Baughman Meditation Center BAU2 1103 GALE LEMERAND DR 1063 Water Reclamation Storage Tank 1103 GALE LEMERAND DR 1064 Hydropneumatic Tank 1103 GALE LEMERAND DR 1065 Chlorine Contact Chamber 1103 GALE LEMERAND DR 1066 Filters 1103 GALE LEMERAND DR 1067 Clarifier (East) 1103 GALE LEMERAND DR 1068 Clarifier (West) 1103 GALE LEMERAND DR 1069 Wwtp Lift Station 1103 GALE LEMERAND DR 1070 Water Reclamation Admin. Bldg. WATR 1103 GALE LEMERAND DR 1071 Water Reclamation Shop/Storage 1103 GALE LEMERAND DR 1073 Water Reclamation Blow/Gen/Elect 1103 GALE LEMERAND DR 1074 Water Reclamation Sludge Bldg 1103 GALE LEMERAND DR 1075 Water Reclamation Electrical 1103 GALE LEMERAND DR 1077 Wwtp Bnr Basins 1103 GALE LEMERAND DR 1078 Wwtp Pretreatment Structure 1104 GALE LEMERAND DR 0963 Parking Garage XIV 1104 Newell Drive 0214 George T. -
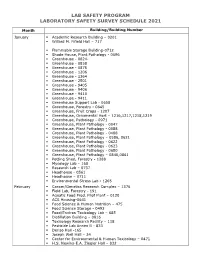
Lab Safety Program Laboratory Safety Survey Schedule 2021
LAB SAFETY PROGRAM LABORATORY SAFETY SURVEY SCHEDULE 2021 Month Building/Building Number January ▪ Academic Research Building – 0201 ▪ Willard M. Fifield Hall – 717 ▪ Flammable Storage Building-0712 ▪ Shade House, Plant Pathology - 0696 ▪ Greenhouse - 0824- ▪ Greenhouse - 0858 ▪ Greenhouse - 0875 ▪ Greenhouse - 1206 ▪ Greenhouse - 1364 ▪ Greenhouse - 2501 ▪ Greenhouse - 9405 ▪ Greenhouse - 9406 ▪ Greenhouse - 9410 ▪ Greenhouse - 9411 ▪ Greenhouse Support Lab - 0658 ▪ Greenhouse, Forestry - 0645 ▪ Greenhouse, Fruit Crops - 1207 ▪ Greenhouse, Ornamental Hort – 1216,1217,1218,1219 ▪ Greenhouse, Pathology - 0971 ▪ Greenhouse, Plant Pathology - 0047 ▪ Greenhouse, Plant Pathology - 0088 ▪ Greenhouse, Plant Pathology - 0488 ▪ Greenhouse, Plant Pathology – 0586, 0621 ▪ Greenhouse, Plant Pathology - 0622 ▪ Greenhouse, Plant Pathology - 0623 ▪ Greenhouse, Plant Pathology - 0650 ▪ Greenhouse, Plant Pathology – 0840,0861 ▪ Potting Shed, Forestry - 1288 ▪ Mycology Lab – 168 ▪ Research Lab – 0737 ▪ Headhouse - 0561 ▪ Headhouse – 0711 ▪ Environmental Stress Lab - 1265 February ▪ Cancer/Genetics Research Complex – 1376 ▪ Field Lab, Forestry - 191 ▪ Aquatic Food Prod. Pilot Plant – 0120 ▪ ACS Housing-0641 ▪ Food Science & Human Nutrition – 475 ▪ Food Science Storage - 0493 ▪ Food/Environ Toxicology Lab – 685 ▪ Distillation Building – 0816 ▪ Toxicology Research Facility – 118 ▪ Pesticide Lab Annex B - 833 ▪ Deriso Hall -165 ▪ Joseph Weil Hall – 24 ▪ Center for Environmental & Human Toxicology – 0471 ▪ H.S. Newins-E.A. Ziegler Hall – 832 LAB SAFETY PROGRAM -

Current Departments NOT Detail Dept Code:
Current Departments NOT Detail Dept Code: - Detail Dept Code: Ascending order Detail Dept Code Detail Dept 00000000 BOARD OF TRUSTEES 00010000 BOT-BRD/TRUSTEES-OFFICE 01000000 OFFICE OF PRESIDENT 01010000 PR-PRESIDENT'S OFFICE 01020000 PR-OFFICE OF INTERNAL AUDIT 02000000 OFFICE OF PROVOST 02010000 PV-VICE PRESIDENT'S OFFICE 02010100 PV-ADMINISTRATION 02010200 PV-ACADEMIC SUPPORT 02010201 PV-ACADEMIC PERSONNEL 02010202 PV-ACADEMIC SUPPORT SVCS 02010203 PV-COMPUTER SUPPORT 02010204 PV-LAN SUPPORT SERVICES 02010205 PV-RECORDS MANAGMENT 02010300 PV-BUDGET OFFICE 02010301 PV-EMPLOYEE EDUC PROGRAM 02010302 PV-HIGHER EDUC OPPORT PROGRAM 02010303 PV-RESERVES 02010400 PV-EARLY CHILDHOOD 02010500 PV-FACULTY AFFAIRS 02010600 PV-INFORMATION TECHNOLOGY 02010601 PV-OAA APPLICATION DEVELOP 02010700 PV-OMBUDS OFFICE 02010800 PV-SENATE CHAIR SUPPORT 02010900 PV-SCHOLARSHIPS / AID 02010901 PV-ALUMINI FELLOWSHIPS 02010902 PV-UNIV SCHOLARS PROGRAM 02010903 PV-FINANCIAL AID 02010904 PV-ACADEMIC SCHOLARSHIPS 02020000 PV-FACULTY DEVELOPMENT 02030000 PV-HONORS OFFICE 02030100 PV-HONORS SCHOLAR PROGRAMS 02040000 PV-AIM PROGRAM 02050000 PV-INSTITUTIONAL RESEARCH 02060000 PV-WRITING PROGRAM 02070000 PV-UNDERGRADUATE STUDIES 02070100 PV-OASIS 02070200 PV-UPWARD BOUND 02070300 PV-CTR FOR UNDERGRAD RESEARCH 02070400 PV-INNOVATION ACADEMY 02090000 PV-UNIV CTR-EXCELLC IN TEACH Sep 16, 2014 - 1 - 10:17:26 AM Current Departments NOT Detail Dept Code: - Detail Dept Code: Ascending order Detail Dept Code Detail Dept 02120000 PV-CNTR PRECOLLEGIATE EDUC 02120100 PV-CPET -

UF Health EDP Dev Vetmed Final Leadership Profile
Executive Director of Development College of Veterinary Medicine Leadership Profile Prepared by Mercedes C. Vance May 2016 This Leadership Profile is intended to provide information about the University of Florida Health and the position of Executive Director of Development, College of Veterinary Medicine. It is designed to assist qualified individuals in assessing their interest in this position. The Opportunity The College of Veterinary Medicine at the University of Florida, one of the nation’s leading veterinary medical colleges, seeks an Executive Director of Development. Reporting to Dean James Lloyd, the executive director will have broad responsibility and authority to build upon past successes in development. Established in 1976, the College of Veterinary Medicine is the state’s only veterinary medical college and offers comprehensive service to the public through a fourfold mission – teaching, research, extension, and patient care. The College of Veterinary Medicine, one of only 30 veterinary medical colleges in the United States, is dedicated to advancing animal, human and environmental health. The college is administered by two long-standing and highly respected University of Florida institutions, the UF Institute of Food and Agricultural Sciences (IFAS) and UF Health. The executive director will oversee a team of six professional staff members in pursuit of increased philanthropic investment. The College of Veterinary Medicine is in the early phases of a $100 million campaign, part of a much larger University initiative, and has already raised $30 million toward that goal. The executive director will oversee the development team and be an experienced development professional with a successful history as a major and principal gift fundraiser. -

CURRICULUM VITAE WESLEY E. BOLCH Professor
CURRICULUM VITAE WESLEY E. BOLCH Professor Department of Biomedical Engineering (www.bme.ufl.edu) 202 Nuclear Science Center, University of Florida Gainesville, Florida 32611‐8300 (352) 392‐1401 ext. 308 (office), (352) 392‐3380 (fax) [email protected] Table of Contents GENERAL INFORMATION 3 Education 3 Professional Certification and Registration 3 Professional and Academic Appointments 3 Significant Honors, Awards, and Appointments 3 Academic Societies 4 Professional Organization – Membership 4 PROFESSIONAL SERVICE 4 Professional Society Activities – Current or Recent 4 Editorial Board Memberships 5 Technical Program Committees 5 Reviews – Journals / Books 5 Reviews – Grants 5 Reviews – Governmental Reports 6 Professional Society Activities – Past 6 University Activities 7 College Activities 7 Departmental Activities 7 CONSULTING 8 PUBLICATIONS 9 Books and Book Chapters 9 Monographs 10 Refereed Journal Articles – Published 10 Refereed Journal Articles – In Press 19 Refereed Journal Articles – Submitted 19 Refereed Journal Articles – In Preparation 19 Proceedings and Transactions – Referred 19 Proceedings and Transactions – Non‐Referred 20 Reviews 21 Reports and Technical Memoranda 21 Miscellaneous Publications 21 Wesley E. Bolch March 13, 2012 Page 1 Table of Contents (continued) PRESENTATIONS 22 International Professional Meeting Presentations – WE Bolch Presenter 22 International Professional Meeting Presentations – Students and Collaborators 24 International Seminars and Lectures – WE Bolch Presenter 26 National Professional Meeting -

MS & Phd in Biostatistics
MS & PhD in Biostatistics Graduate Student Handbook (July 2015) Department of Biostatistics College of Public Health and Health Professions College of Medicine http://www.biostat.ufl.edu Biostatistics PhD Handbook Table of Contents Page Introduction 3 Part I: The University of Florida 3 • Overview of UF 4 • The Health Science Center 5 • College of Public Health and Health Professions 7 • College of Medicine 8 • Department of Biostatistics 8 Part II: PhD in Biostatistics 9 • Program Overview 9 • Student Learning Outcomes 10 • Administration and Faculty 10 • Student Guidance and Mentoring 14 • Supervisory Committee 15 • Curriculum 16 • Registration and Credit Transfers 19 • Qualifying Examination 21 • Admission to Candidacy 22 • Dissertation 23 • Final Dissertation Defense 24 • Submission of the Dissertation 25 • Sequence of Major Events 26 Part III: MS in Biostatistics 27 • Masters in Biostatistics Curriculum Overview 28 • Consulting Requirement 29 • Comparison to M.S. in Statistics 29 • M.S. Advisor 29 • Capstone Experience 30 Part IV: Important Information 30 • GatorLink 30 • Financial Aid 31 • Florida Residency 31 • Academic Integrity 32 • Grievance Procedures 33 • Readmission Requirements 34 • Libraries 35 • Computer Policy 35 Part V: Major Research Centers 37 • Clinical and Translational Science Institute 37 • Emerging Pathogens Institute 38 • Florida Center for Medicaid and the Uninsured 39 • Institute on Aging 39 • Institute for Child Health Policy 40 • McKnight Brain Institute 40 • UF Genetics Institute 41 • UF Health Cancer Center 41 Appendix: Department Forms 42 Introduction Revised 8/5/2015 Page 2 Biostatistics PhD Handbook This handbook is intended to provide direction for you as you navigate through the Biostatistics MS and PhD Programs. Most of these requirements have been established by the Graduate School http://gradschool.ufl.edu/, the University of Florida, or the UF Board of Trustees. -
Tenure Track Faculty Positions in Biological Physics at the University of Florida
Tenure Track Faculty Positions in Biological Physics at the University of Florida As part of a major, campus-wide faculty hiring initiative the Department of Physics at the University of Florida (UF) seeks to fill multiple nine-month, full-time, tenure-track Assistant Professor positions in biological physics, to begin August 2019. Applications are encouraged from highly qualified candidates in the following areas: Experimental biological physics, including but not limited to molecular and cellular function and dynamics, systems biology, networks, development, signaling, information processing, and related topics. Apply online at https://apply.interfolio.com/55533. For more information contact the Chair of the Experimental Biophysics Search at [email protected] Theoretical biological physics, including but not limited to molecular and cellular function and dynamics, systems biology, biological soft matter, bioinformatics and related areas. Apply online at https://apply.interfolio.com/55601. For more information contact the Chair of the Theoretical Biophysics Search at [email protected] Computational biological physics, including but not limited to exploration of quantum mechanical, atomistic, or multiscale simulations of biomaterials and biomolecules and other processes in biomolecular and biochemical systems. Apply online at https://apply.interfolio.com/55788. For more information contact the Chair of the Computational Biophysics Search at [email protected] Applications must be submitted online and must include: (i) cover letter, (ii) curriculum vitae with full publication list, (iii) statement of research and proposed activities, (iv) statement of teaching philosophy, (v) three confidential letters of recommendation. To ensure full consideration, applications must be submitted by November 15, 2018. -

(ARML) Program and Cognitive Aging and Memory (CAM) Program 2016 Annual Report
Age-related Memory Loss (ARML) Program and Cognitive Aging and Memory (CAM) Program 2016 Annual Report Prepared for the McKnight Brain Research Foundation by the University of Florida McKnight Brain Institute and Institute on Aging Table of Contents Letters from UF/MBI Leadership .................................................................................................................................................................................. 3-7 Age-related Memory Loss (ARML) Program and the Evelyn F. McKnight Chair for Brain Research in Memory Loss...... 8-25 Cognitive Aging and Memory Clinical Translational Research Program (CAM-CTRP) and the Evelyn F. McKnight Chair for Clinical Translational Research in Cognitive Aging ....................................................... 26-59 William G. Luttge Lectureship in Neuroscience ...............................................................................................................60-61 Faculty Biographical Sketches ........................................................................................................................................ 62-184 Selected Publications .................................................................................................................................................... 185-315 Offi ce of UF Senior Vice President for Health Aff airs 1515 SW Archer Road, Suite 23C1 Gainesville, FL 32608 P.O. Box 100014 Gainesville, FL 32610-0014 Phone: 352.733.1700 Fax: 352.733.1201 UFHealth.org Patient Care ● Research ● Education -

The UF Campus
Welcome to the UF Campus The places you’ll visit today have one thing in common: Each reflects our core commitments to excellence, learning, internationalization and interdisciplinary endeavors. They are woven throughout all our efforts and interconnect as they span our unique program array. Excellence in all areas personifies UF. From our academic rankings, research figures, athletic championships to our national and international reach, our commitment to excellence is reflected in everything we do. It’s in our DNA. Learning embodies everything we do, from research and teaching to public service. From our strong support of faculty — through endowments and the recent creation of more than 100 new endowed faculty chairs — to the success of our graduates as sought- after recruits, a relentless focus on new knowledge and the value of a UF education drives us daily. The University of Florida is “International by Intent”. We have made a conscious effort to enhance the educational experience and environment of our faculty, students and staff by promoting a global perspective. Nearly 140 countries are represented in the UF student body. Diversity is a proud part of our identity, and contributes to the excellence of our innovative work in all fields. It takes an institution with our depth, breadth and scope to truly deliver on the promise and potential of interdisciplinary research and teaching. UF is a national leader in leveraging interdisciplinary collaboration to change the way people experience their lives. UF’s Cultural Heart THE CULTURAL PLAZA The Phillips Center for the Performing Arts, the Harn Museum of Art and the Florida Museum of Natural History are dedicated to celebrating multiple art forms, presenting established and emerging national and international artists and preserving and interpreting biological diversity and cultural heritage. -
University of Florida Board of Trustees Committee on Facilities and Capital Investments Committee Action Item Fci June 6, 2019
UNIVERSITY OF FLORIDA BOARD OF TRUSTEES COMMITTEE ON FACILITIES AND CAPITAL INVESTMENTS COMMITTEE ACTION ITEM FCI JUNE 6, 2019 SUBJECT: University of Florida Educational Plant Survey Validation BACKGROUND INFORMATION 1. An Educational Plant Survey (EPS) is required once every five (5) years for all public educational entities, including state universities. At the request of the University of Florida (UF), Board staff facilitated and coordinated the Survey Team and participated with university staff on the EPS to ensure that all the requirements of section 1013.31, Florida Statues, were satisfied. In addition to UF and Board staff, the team included staff from University of West Florida, University of Central Florida, University of North Florida, Florida Agricultural and Mechanical University, and Florida Gulf Coast University. The Survey Team Recommendation is included as an attachment. The EPS covers the period July 1, 2019 through June 30, 2024, and is UF’s first EPS completed using the Dynamic Capital Planning (DCP) model. PROPOSED COMMITTEE ACTION Approve the review and validation of the completed University of Florida Educational Plant Survey. SIGNIFICANT POLICY ISSUES FOR COMMITTEE TO CONSIDER Article IX, Section 7, Florida Constitution; Sections 1013.03 and 1013.31, Florida Statues Supporting Documentation Included: Summary of Survey Team Recommendations, and the Educational Plant Survey Packet. Submitted by: Curtis A. Reynolds, VP, Business Affairs RECOMMENDATIONS OF SURVEY TEAM University of Florida Needs Assessment Date: April 24, 2019. Survey Team Members: Robin Anderson, Team Leader (UWF), Kenneth Ogletree (BOG), Felcy Gabriel (BOG), Kristine Azzato (BOG), Mary Mory (UNF), Takeidra Nelson (FAMU), Krystie Corbitt (FGCU), Christy Miranda (UCF) Site Improvements Recommendations: 1.1 Land Acquisition – Not Applicable 1.2 Landscaping and Site Improvements – This is a recommendation to continue landscaping and site improvements consistent with the adopted Campus Master Plan. -

University of Florida College of Medicine
UNIVERSITY OF FLORIDA COLLEGE OF MEDICINE Education The College of Medicine is the largest college within the University of Florida Health Science Center and one of the largest colleges within the university as a whole. Its mission is to improve health care in Florida, the nation and the world through excellence and leadership in education, clinical care, education, discovery and service. Established in 1956, the college was one of the first in Florida to provide students with the opportunity to earn a medical degree. The college is ranked No. 43 among the nation’s top research medical schools, according to U.S. News & World Report. Among public medical schools, UF ranks No. 17 nationally and is the highest-ranked medical school in the state of Florida. In July 2015, the college opened the George T. Harrell, M.D., Medical Education Building. Michael L. Good, M.D., Designed to support the college’s updated Dean, College of Medicine medical education curriculum, the building Folke H. Peterson Dean’s Distinguished Professor provides a dynamic environment for all learners as they develop the skills necessary to meet society’s health care needs and to ensure the highest level of patient care. 28 In addition to medical training, the college offers research-oriented basic and clinical academic a variety of educational opportunities, including departments the Interdisciplinary Program in Biomedical Sciences, which leads to a Ph.D. or an M.S. degree, and joint programs for M.D. and Ph.D. degrees. The College of Medicine is also home to the School of Physician Assistant Studies.