State Ex Rel. Jennings V. Purdue Pharma LP, Not Reported in Atl. Rptr
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cardinal Health 2019 Annual Report
2019 Annual Report Essential to care ™ Corporate Offices Cardinal Health 7000 Cardinal Place Dublin, Ohio 43017 614.757.5000 www.cardinalhealth.com © 2019 Cardinal Health. All Rights Reserved. CARDINAL HEALTH, the Cardinal Health LOGO and ESSENTIAL TO CARE are trademarks of Cardinal Health and may be registered in the US and/or in other countries. All other marks are the property of their respective owners. Lit. No. 5CR19-971925 (08/2019) DearDear fellow fellow shareholders: shareholders: CorporateCorporate and and In myIn my letter letter to toyou you last last year, year, I acknowledged I acknowledged investorinvestor information information thethe trust trust you you place place in usin usto torun run the the business business CorporateCorporate offices offices FinancialFinancial information information effectivelyeffectively and, and, in inturn, turn, be beresponsible responsible CardinalCardinal Health Health ComprehensiveComprehensive financial financial and andother other information information about about stewardsstewards of ofyour your capital. capital. I’d I’dlike like to toshare share with with 70007000 Cardinal Cardinal Place Place CardinalCardinal Health Health can can be obtainedbe obtained by visiting by visiting the theInvestor Investor Dublin,Dublin, Ohio Ohio 43017 43017 RelationsRelations page page at ir.cardinalhealth.com. at ir.cardinalhealth.com. youyou some some of ofour our accomplishments accomplishments over over 614.757.5000614.757.5000 thethe past past year year and and where where we we are are going going -

Catalent, Inc. Annual Report 2019
Catalent, Inc. Annual Report 2019 Form 10-K (NYSE:CTLT) Published: August 27th, 2019 PDF generated by stocklight.com UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 ______________________________ FORM 10-K ______________________________ x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended June 30, 2019 or o TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from to Commission File Number: 001-36587 ___________________________ CATALENT, INC. (Exact name of registrant as specified in its charter) ______________________________ Delaware 20-8737688 (State or other jurisdiction of incorporation or organization) (I.R.S. Employer Identification No.) 14 Schoolhouse Road 08873 Somerset, New Jersey (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code: (732) 537-6200 ______________________________ Securities registered pursuant to Section 12(b) of the Act: Title of each class Trading Symbol(s) Name of each exchange on which registered Common Stock, $0.01 par value per share CTLT New York Stock Exchange Securities registered pursuant to Section 12(g) of the Act: None ______________________________ Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes x No o Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Cardinal Health 2018 Proxy Statement
2018 Proxy Statement Notice of Annual Meeting of Shareholders Letter to Cardinal Health Shareholders 1 Notice of Annual Meeting of Shareholders 3 Proxy Summary 4 Fiscal 2018 Highlights 4 Governance and Board Highlights 4 Our 2018 Board Nominees 5 Addressing the Opioid Crisis 6 Attending the Annual Meeting of Shareholders 8 Roadmap to Voting Matters 8 How to Vote 8 Corporate Governance 9 Proposal 1 — Election of Directors 9 Board Membership Criteria: What we look for 9 Our Director Nominees 9 Our Board’s Composition and Structure 15 Our Board’s Primary Role and Responsibilities and Processes 19 Shareholder Engagement 21 Director Compensation 22 Related Person Transactions Policy and Process 23 Audit Committee Matters 24 Proposal 2 — Ratification of Appointment of Ernst & Young LLP as Independent Auditor 24 Audit Committee Report 24 Fees Paid to Ernst & Young LLP 25 Policy on Pre-Approval of Services Provided by Ernst & Young LLP 25 Executive Compensation 26 Proposal 3 — Advisory Vote to Approve the Compensation of Our Named Executive Officers 26 Compensation Discussion and Analysis 27 Human Resources and Compensation Committee Report 34 Executive Compensation Tables 35 Pay Ratio Disclosure 48 Shareholder Proposals 49 Proposal 4 — Shareholder Proposal on a Policy to Not Exclude Legal and Compliance Costs for Purposes of Determining Executive Compensation 49 Proposal 5 — Shareholder Proposal on the Ownership Threshold for Calling a Special Meeting of Shareholders 51 Share Ownership Information 52 Beneficial Ownership 52 Compliance with Section 16(a) of the Exchange Act 53 Other Matters 54 General Information About the Annual Meeting of Shareholders 54 Communicating with the Board 56 Shareholder Recommendations for Director Nominees 56 Submitting Proxy Proposals and Director Nominations for the Next Annual Meeting of Shareholders 56 Corporate Governance Guidelines 57 Transfer Agent 57 Other Information 57 Annex A — Use of Non-GAAP Financial Measures 58 strong leadership and commitment to Cardinal Health during his Letter to Cardinal tenure. -

Cardinal Health, Inc. (Exact Name of Registrant As Specified in Its Charter)
Table of Contents UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Form 10-Q QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the quarterly period ended December 31, 2016 or TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the transition period from ________ to ________ Commission File Number: 1-11373 Cardinal Health, Inc. (Exact name of registrant as specified in its charter) Ohio 31-0958666 (State or other jurisdiction of (IRS Employer incorporation or organization) Identification No.) 7000 Cardinal Place, Dublin, Ohio 43017 (Address of principal executive offices) (Zip Code) (614) 757-5000 (Registrant’s telephone number, including area code) Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes No Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes No Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. -

2008 Annual Report
essential to care Annual Report 2008 In today’s complex world of healthcare, safe and efficient care is not optional. It is essential. At Cardinal Health, we work with hospitals and pharmacies across the globe every day to improve the safety and productivity of the care they provide. Long before a patient arrives at a hospital for treatment or calls a pharmacy for a prescription, Cardinal Health is an essential part of delivering safe and eff icient care. We provide the medical technologies that help ensure safe doses of critical medications at the bedside. Our surgical gloves help protect both caregivers and patients from infections. We deliver medicine to pharmacies, so it is ready to be dispensed at a moment’s notice. These are just a few examples of how Cardinal Health is essential to care. But it is not enough to provide the products and services that are essential in today’s healthcare system. At Cardinal Health, essential to care means: Caring about our customers. Caring about patient outcomes. Caring about employees. Caring about shareholders. Caring about our communities. Essential to care. It is the driving force behind Cardinal Health’s mission of making healthcare safer and more productive. Chairman’s letter To our customers, employees and shareholders: In the United States and around the world, the cost of to do: help sick patients get better. That’s why we believe delivering healthcare continues to accelerate. I believe that that no company is better positioned than Cardinal Health one of the keys to driving a meaningful reduction in the cost to streamline the healthcare supply chain, help clinicians of care is improving the quality of care. -

Cardinal Health 2020 Annual Report
2020 Annual Report Dear fellow shareholders, A year ago, I closed my letter to you with a reflection on our role in healthcare. Over the past several months, we have seen that now more than ever, what we do matters — to our customers, to the patients they serve, and to our communities. In my nearly 30 years as part of the Cardinal Health family, I have experienced significant transformation, both within our company and within the healthcare industry. We have expanded our products, services and markets, evolved with technological and regulatory changes, and adapted in the face of external challenges. In each of these moments, we have demonstrated agility and embraced change to emerge as a stronger company with an even more solidified role in healthcare. Now, as we navigate the unprecedented challenges of a global pandemic, we are leaning on that legacy of adaptability and dedication. In fiscal 2020, we delivered on our commitments and continued to execute on our long-term strategic priorities, all as we adapted our operations to address the unique challenges presented by COVID-19. Our fiscal 2020 financial performance Across the company, in fiscal 2020, we demonstrated positive In the Medical segment, our cost savings initiatives, performance despite significant global challenges. We grew particularly within our global manufacturing and supply non-GAAP operating earnings, exceeded our non-GAAP chain, drove significant benefits throughout the year. We EPS guidance range, surpassed our cost savings target, and expect these workstreams to continue to deliver greater strengthened our balance sheet. In the year, we invested $375 efficiencies and additional value into the future. -

Corporate Citizenship Report 2019 Table of Contents
Corporate Citizenship Report 2019 Table of contents Editor’s note: This report was largely created before the COVID-19 pandemic. During the pandemic, we at Cardinal Health are working around the clock to meet the needs of healthcare providers so they can safely serve the patients who depend on them. Learn about our response » A message to our stakeholders ______________________________ 3 Creating value for our customers and our partners ����������27 Essential to care™ _________________________________________ 7 Strengthening our communities _________________________41 Our Path Forward ________________________________________ 8 Operating sustainably and responsibly ____________________48 ESG management and governance __________________________ 9 Governance and ethics _________________________________56 Stakeholder engagement _________________________________ 11 About this report _______________________________________ 63 Empowering our people ________________________________ 12 GRI index _____________________________________________ 64 Table of contents Our people Customers and partners Our communities Sustainability Governance GRI index 2019 Corporate Citizenship Report | 2 A message to our stakeholders This Corporate Citizenship Report was nearly complete when the COVID-19 pandemic changed the world in ways no one could ever have imagined. So I am beginning this letter differently than I originally planned. First, I want to express my deepest gratitude to every frontline worker and healthcare professional around the globe. The courage and commitment you bring to work with you every day is awe-inspiring. Your work is making a difference. Thank you. Since early March, the 48,000 Cardinal Health employees around the we have significantly expanded our technology infrastructure to help world have been more focused than ever before on delivering the employees around the globe continue to support customers, patients products and solutions that improve the lives of people every day. -

Distributors New York Settlement Agreement
DISTRIBUTORS NEW YORK SETTLEMENT AGREEMENT Table of Contents I. Overview ......................................................................................................................1 II. Definitions ....................................................................................................................1 III. Condition to Effectiveness of Agreement ..................................................................12 IV. Participation by Subdivisions .....................................................................................12 V. Settlement Payments ..................................................................................................14 VI. Allocation and Use of Settlement Payments. .............................................................26 VII. Enforcement ...............................................................................................................27 VIII. Plaintiffs’ Attorneys’ Fees and Costs .........................................................................27 IX. New York Additional Restitution Amount ................................................................30 X. Release ........................................................................................................................30 XI. Later Litigating Subdivisions .....................................................................................34 XII. Reductions/Offsets .....................................................................................................38 XIII. Injunctive Relief -

Pharma Services Q2 2019
June 2019 CDMO – Transaction Comps CDMO - Transaction Comps USD in millions Announced Geographic EV / LTM EV / LTM Date Target Target Description Buyer Location Enterprise Value LTM Revenue LTM EBITDA Revenue EBITDA Operates as a CDMO that focuses on the development and Apr-19 Paragon Bioservices1 Catalent Pharma Solutions USA $1,200.0 $101.0 $56.0 11.9x 21.4x manufacturing of biopharmaceuticals Biogen (Denmark) Biopharmaceutical CDMO facility with 90,000L biologic Fujifilm Diosynth Mar-19 Denmark 890.0 NA NA NA NA Manufacturing Facility production capacity Biotechnologies Mar-19 Brammer Bio CDMO developing and manufacturing cell and gene therapies Thermo Fisher USA 1,700.0 NA NA NA NA CDMO offering services including analytical testing, API and Nov-18 Avista Pharma Solutions2 drug product development, early stage discovery, and Cambrex Corporation USA 252.0 65.0 NA 3.9x NA microbiology testing support Operates as a custom manufacturer of APIs and registered Jul-18 AMPAC Fine Chemicals3 SK Holdings USA 455.0 200.0 NA 2.3x NA intermediates for the pharmaceutical industry Provides contract development and manufacturing services in Jul-18 Halo Pharma Cambrex Corporation USA 425.0 105.0 27.0 4.0x 15.7x various dosage forms and drug delivery platforms Provides contract development and manufacturing services to Sep-17 Cook Pharmica Catalent Pharma Solutions USA 950.0 177.8 NA 5.3x NA pharmaceutical and biopharmaceutical companies Provides drug discovery and development services, as well as Jul-17 Aptuit Evotec USA 300.0 92.9 11.6 3.2x 25.8x -
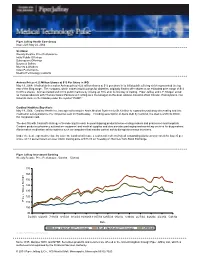
Since 1895. Member SIPC and NYSE. Securities Products And
Piper Jaffray Health Care Group Issue 229: May 24, 2004 Sections: Weekly Relative Price Performance Initial Public Offerings Subsequent Offerings Buyers & Sellers Movers & Shakers Index Performance Medical Technology Contacts Animas Prices 4.25 Million Shares at $15 Per Share in IPO May 19, 2004: Medical device maker Animas priced 4.25 million shares at $15 per share in its initial public offering which represented the top end of the filing range. The company, which makes insulin pumps for diabetics, originally filed to offer shares at an estimated price range of $13 to $15 a shares. Animas traded well in the public markets by closing up 18% after its first day of trading. Piper Jaffray and J.P. Morgan acted as co-lead advisors with Thomas Weisel Partners LLC acting as a co-manager on the deal. Animas, based in West Chester, Pennsylvania, has listed its stock on the Nasdaq under the symbol "PUMP." Cardinal Health to Buy Alaris May 19, 2004: Cardinal Health Inc. has agreed to acquire Alaris Medical Systems for $1.6 billion to expand beyond drug wholesaling and into medication safety products, the companies said on Wednesday. Including assumption of Alaris debt by Cardinal, the deal is worth $2 billion, the companies said. The deal fits with Cardinal's strategy of broadening its reach beyond shipping products between drug makers and pharmacies and hospitals. Cardinal produces pharmacy automation equipment and medical supplies and also provides packaging and marketing services for drug makers. Alaris makes medication safety systems such as computers that monitor patient safety during intravenous injections. -

Carefusion Corporation (Exact Name of Registrant As Specified in Its Charter)
As filed with the Securities and Exchange Commission on March 31, 2009 File No. SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 Form 10 GENERAL FORM FOR REGISTRATION OF SECURITIES Pursuant to Section 12(b) or (g) of the Securities Exchange Act of 1934 CareFusion Corporation (Exact name of Registrant as specified in its charter) Delaware 26-4123274 (State or other jurisdiction of (I.R.S. employer incorporation or organization) identification number) 3750 Torrey View Court, San Diego, CA 92130 (Address of principal executive offices) (Zip Code) (858) 617-2000 (Registrant’s telephone number, including area code) Securities to be registered pursuant to Section 12(b) of the Act: Title of Each Class to be so Registered Name of Each Exchange on which Each Class is to be Registered Common Stock, par value $0.01 per share New York Stock Exchange Securities to be registered pursuant to Section 12(g) of the Act: None CAREFUSION CORPORATION INFORMATION REQUIRED IN REGISTRATION STATEMENT CROSS-REFERENCE SHEET BETWEEN INFORMATION STATEMENT AND ITEMS OF FORM 10 Certain information required to be included herein is incorporated by reference to specifically identified portions of the body of the information statement filed herewith as Exhibit 99.1. None of the information contained in the information statement shall be incorporated by reference herein or deemed to be a part hereof unless such information is specifically incorporated by reference. Item 1. Business. The information required by this item is contained under the sections of the information statement entitled “Information Statement Summary,” “Business” and “Our Relationship with Cardinal Health Following the Distribution.” Those sections are incorporated herein by reference. -

Top 2000 Valued Companies with Ticker Symbols.Pdf
Name Symbol Market Value (millions) Exxon Mobil Corporation XOM $384,845.80 General Electric Company GE $346,042.10 Microsoft Corporation MSFT $299,647.60 BP p.l.c. BP $248,469.70 Citigroup, Inc. C $231,977.70 Procter & Gamble Company (The) PG $194,814.00 Wal-Mart Stores, Inc. WMT $191,978.50 Pfizer, Inc. PFE $189,297.40 HSBC Holdings, plc. HBC $188,804.60 Toyota Motor Corporation TM $187,196.40 JOHNSON & JOHNSON JNJ $179,515.80 Bank of America Corporation BAC $176,906.20 American International Group, Inc. AIG $169,908.50 TotalFinaElf, S.A. TOT $169,852.60 Novartis AG NVS $151,092.70 Altria Group MO $150,638.60 GLAXOSMITHKLINE PLC GSK $149,062.80 Mitsubishi UFJ Financial Group Inc MTU $146,421.90 J.P. Morgan Chase & Co. JPM $138,596.30 ROYAL DUTCH SHELL PLC RDS/A $133,773.40 ChevronTexaco Corporation CVX $133,307.10 Sanofi-Aventis SA SNY $128,920.20 Vodafone AirTouch Public Limited Company VOD $128,885.40 Intel Corporation INTC $128,146.40 International Business Machines Corporation IBM $127,641.00 ENI S.p.A. E $121,462.50 Cisco Systems, Inc. CSCO $114,067.10 Berkshire Hathaway Inc. BRK/A $112,846.90 UBS AG UBS $110,546.60 Wells Fargo Cap IX WFC $104,281.10 AT&T Inc. T $101,549.80 ROYAL DUTCH SHELL PLC RDS/B $99,061.00 Coca-Cola Company (The) KO $98,450.10 China Mobile (Hong Kong) Ltd. CHL $97,501.20 Pepsico, Inc. PEP $94,976.00 Verizon Communications Inc.