148. Critical Review of Biology And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Severe Introduced Predator Impacts Despite Attempted Functional Eradication
bioRxiv preprint doi: https://doi.org/10.1101/2021.07.19.451788; this version posted July 19, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Severe introduced predator impacts despite attempted functional eradication 2 Brian S. Cheng1,2, Jeffrey Blumenthal3,4, Andrew L. Chang3,4, Jordanna Barley1, Matthew C. Ferner4,5, Karina J. Nielsen4, Gregory M. Ruiz2, Chela J. Zabin3,4 4 1Department of Environmental Conservation, University of Massachusetts Amherst, Amherst, MA 01002 6 2Smithsonian Environmental Research Center, Edgewater, MD, 21037 3Smithsonian Environmental Research Center, Tiburon, CA 94920 8 4Estuary & Ocean Science Center, San Francisco State University, Tiburon, CA 94920 5San Francisco Bay National Estuarine Research Reserve, Tiburon, CA 94920 10 ORCID: BSC 0000-0003-1679-8398, ALC 0000-0002-7870-285X, MCF 0000-0002-4862-9663, KJN 0000-0002-7438-0240, JGB 0000-0002-8736-7950, CJZ 0000-0002-2636-0827 12 DECLARATIONS 14 Funding: Advancing Nature-Based Adaptation Solutions in Marin County, California State Coastal Conservancy and Marin Community Foundation. 16 Conflicts/Competing interests: The authors declare no conflicts or competing interests. Availability of data and code: All data and code has been deposited online at 18 https://github.com/brianscheng/CBOR Ethics approval: not applicable 20 Consent to participate: not applicable Consent for publication: not applicable 22 Page 1 of 37 bioRxiv preprint doi: https://doi.org/10.1101/2021.07.19.451788; this version posted July 19, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. -

January-February (1.58 Mbs)
January-February, 2012 __________________________________________________ Volume 53 no. 1 The club will meet at the usual time (7:00 PM) and place (SW Branch Jacksonville Public Library), and we have reprised the time-honored monthly date, the fourth Thursday (Jan. 26, 2012). The program will be presented by Charlotte Thorpe and Harry Lee on the club field trip to Cedar Key the weekend after Thanksgiving. Following the presentation there will be time allotted for show-and-tell as well as an ID clinic, so bring any west FL shells you might find of interest to the group and/or in need of identification. Brian Marshall will open the meeting with a chronicle of the New Years Day hunt for the first living specimen of the Panasoffkee Liptooth, the Shell-of-the-Month and a snail new to science. On February 23 we will convene at the usual time and place. On tap will be a presentation by Rick Edwards, who, with Roz and their son William, enjoyed a Caribbean cruise including Grand Cayman Island and Cozumel. He was able to get his feet in the water in both ports-of-call, and his underwater camera was fully operational. Harry Lee will talk about how he deployed pharmaceutical rep's to collect marine sediments in Grand Cayman and other Caribbean waters. A recently-named micromollusk provided by this armchair collecting method will be featured. ......................................................................................................................................................................... Eighteen Jacksonville Shell Club Members attended the Christmas Party hosted by Claire Newsome on December 10th. We enjoyed a wonderful dinner, a fun gift exchange, companionship, and a "Good Time" was had by all. -

History of Methodism in This Territory Should Be Prepared Especially Dealing with the Past fifty Years Extending from the ’ Date at Which the Late Dr
H I S T O R Y O F ME T HODI SM E ASTE RN BRI TI SH AME RI CA I NCLUDING NOVA SCO TI A, NE W BRUNSWICK PRINCE EDWARD ISLAND NEWFOUNDLAND AND BERMUDA FROM THE BE GI NNI NG TI LL THE CONSUMMATI ON OF U NI ON WI TH THE PRE SBYT E RI AN AND CONGRE GATI ONAL CHURCHE S I N 1925 BY D . W. JOHNS ON ' ‘ OF THE - NOVA SC OTI A CONFERENCE, E X E DI I OB OF THE WESLEYAN C O N T E N T S C h a pte r 1 Th e G e n e si s of Me h o m . t d i s 2 No a Sco on f e r . v tia C e n c e f ol l owin g c ircu it o rd e r a s in Ye a r Book o f 1924 Ne w B un s wi ck a n d Prin ce E dwa s l an d n cl ud n Bib l e r rd I , i i g h s ia n s C in P E . ri t . I N e wfoun d an d Co n fe r e n ce in lu d n L ab ad an E d uc a on l , c i g r or d ti in N e wfoun dl a n d Me th od i s m in Be rmud a Moun t Al llis on In s titution s We s l e ya n a n d Boo k Room C hu rch U n io n ’ Wo man s Mi s s i on a ry So c ie ty Ho m e a n d Fore i g n Mis sion s Ap p e n d ice s T h e C o n s tituti on an d P e r son n e l o f th e E a s te rn British Ame r ica Co n fe re n c e —18 55 to 18 74“ N ova fSco tia C on fe re n c e n am e s an d fig ur e s N B a n d .P E I Con f e e n c me s a n d ur e s . -

C S a S S C É S Canadian Stock Assessment Secretariat Secrétariat Canadien Pour L’Évaluation Des Stocks
Fisheries and Oceans Pêches et Océans Science Sciences C S A S S C É S Canadian Stock Assessment Secretariat Secrétariat canadien pour l’évaluation des stocks Document de recherche 2000/007 Research Document 2000/007 Not to be cited without Ne pas citer sans permission of the authors 1 autorisation des auteurs 1 Atlantic salmon (Salmo salar L.) stock status on rivers in the Northumberland Strait, Nova Scotia area, in 1999 S. F. O’Neil, K.A. Rutherford, and D. Aitken Diadromous Fish Division Science Branch, Maritimes Region Bedford Institute of Oceanography P.O.Box 1006 Dartmouth, N.S. B2Y 4A2 1 This series documents the scientific basis for 1 La présente série documente les bases the evaluation of fisheries resources in scientifiques des évaluations des ressources Canada. As such, it addresses the issues of halieutiques du Canada. Elle traite des the day in the time frames required and the problèmes courants selon les échéanciers documents it contains are not intended as dictés. Les documents qu’elle contient ne definitive statements on the subjects doivent pas être considérés comme des addressed but rather as progress reports on énoncés définitifs sur les sujets traités, mais ongoing investigations. plutôt comme des rapports d’étape sur les études en cours. Research documents are produced in the Les documents de recherche sont publiés dans official language in which they are provided to la langue officielle utilisée dans le manuscrit the Secretariat. envoyé au Secrétariat. This document is available on the Internet at: Ce document est disponible sur l’Internet à: http://www.dfo-mpo.gc.ca/csas/ ISSN 1480-4883 Ottawa, 2000 i Abstract Fifteen separate rivers on the Northumberland Strait shore of Nova Scotia support Atlantic salmon stocks. -

Atlantic Salmon Stock Status on Rivers in the Northumberland Strait, Nova
Department of Fisheries and Oceans Ministère des pêches et océan s Canadian Stock Assessment Secretariat Secrétariat canadien pour l'évaluation des -stocks Research Document 97/22 Document de recherche 97/22 Not to be cited without Ne pas citer sans permission of the authors ' autorisation des auteurs ' Atlantic salmon (Salmo salar L .) stock status on rivers in the Northumberland Strait, Nova Scotia area, in 1996 S. F. O'Neil, D. A. Longard, and C . J. Harvie Diadromous Fish Division Science Branch Maritimes Region P.O.Box 550 Halifax, N .S. B3J 2S7 ' This series documents the scientific basis for ' La présente série documente les bases the evaluation of fisheries resources in Canada . scientifiques des évaluations des ressources As such, it addresses the issues of the day in halieutiques du Canada. Elle traite des the time frames required and the documents it problèmes courants selon les échéanciers contains are not intended as definitive dictés. Les documents qu'elle contient ne statements on the subjects addressed but doivent pas être considérés comme des rather as progress reports on ongoing énoncés définitifs sur les sujets traités, mais investigations . plutôt comme des rapports d'étape sur les études en cours. Research documents are produced in the Les documents de recherche sont publiés dans official language in which they are provided to la langue officielle utilisée dans le manuscrit the Secretariat . envoyé au secrétariat . ii Abstrac t Fifteen separate rivers on the Northumberland Strait shore of Nova Scotia support Atlantic salmon stocks. Stock status information for 1996 is provided for nine of those stocks based on the conservation requirements and escapements calculated either from mark-and-recapture experiments (East River, Pictou and River Philip) or capture (exploitation) rates in the angling fishery. -

The American Oyster. INSTITUTION, Maryland Univ., College Park
DOCUMEk RESUME ED 225 840 SE 040 199 AUTHOR Thompson, Nancy E. TITLE The American Oyster. INSTITUTION, Maryland Univ., College Park. Sea GrantProgram. SPONS AGENCY National Oceanic and AtmosphericAdministration .(DOC), Rockville, Md. National Sea Grant Program. REPORT NO UM-SG-ES-79-03 PUB DATE 79 GRANT NA81AA-D-00040 NOTE AVAILABLE FROMUniversity of Maryland Sea Grant Program, H.J. Patterson Hall, College Park, MD20742 ($2.00). PUB TYPE Guides Classroom Use - Guides (For Teachers)(052) EDRS PRICE MF01/PC03 Plus Postage. DESCRIPTORS *Animals; *Biological Sciences; Classification; Ecology; Elementary School Science;Elementary Secondary Education; Environmental Education;*Marine Biology; *Science Activities; ScienceEducation; *Secondary School Science; Wildlife IDENTIFIERS Estuaries; *Marine Education; *Oysters ABSTRACT The Maryland Marine ScienceEducatiOn Project has produced a series of mini-units inmarine science education for the junior high/middle school classroom.This unit focuses on the American oyster. Although the unitspecifically treats the Chesapeake .Bay, it may be adapted for usewith similar estuarine systems. In addition, the unit may be incorporatedinto existing life science courles using the Chesapeake Bay as a concrete example ofworking biological principles. The unitconsists of sections devoted to content/background reading for the teacher,student activities, and resource materials. Topicsin the teacher's narrative include background information on the ChesapeakeBay, nature/anatomy of oysters, oyster life cycle, oysterpredators/parasites, oyster distribution and possib,le reasons for theirdecline, methods of oyster harvest in the Chesapeake, stateoyster repletion program, and comments on oyster processing.Eight activities/games are included in the student activities section.These activities include graph interpretation, map reading, oyster classification,making pyster stew, and three vocabulary games(bingo, rummy, and a word search). -

Malacofauna Marina Del Parque Nacional “Los Caimanes”, Villa Clara, Cuba
Tesis de Diploma Malacofauna Marina del Parque Nacional “Los Caimanes”, Villa Clara, Cuba. Autora: Liliana Olga Quesada Pérez Junio, 2011 Universidad Central “Marta Abreu” de Las Villas Facultad Ciencias Agropecuaria TESIS DE DIPLOMA Malacofauna marina del Parque Nacional “Los Caimanes”, Villa Clara, Cuba. Autora: Liliana Olga Quesada Pérez Tutor: M. C. Ángel Quirós Espinosa Investigador Auxiliar y Profesor Auxiliar [email protected] Centro de Estudios y Servicios Ambientales, CITMA-Villa Clara Carretera Central 716, Santa Clara Consultante: Dr.C. José Espinosa Sáez Investigador Titular Instituto de Oceanología Junio, 2011 Pensamiento “La diferencia entre una mala observación y una buena, es que la primera es errónea y la segunda es incompleta.” Van Hise Dedicatoria Dedicatoria: A mis padres, a Yandy y a mi familia: por las innumerables razones que me dan para vivir, y por ser fuente de inspiración para mis metas. Agradecimientos Agradecimientos: Muchos son los que de alguna forma contribuyeron a la realización de este trabajo, todos saben cuánto les agradezco: Primero quiero agradecer a mis padres, que aunque no estén presentes sé que de una forma u otra siempre estuvieron allí para darme todo su amor y apoyo. A mi familia en general: a mi abuela, hermano, a mis tíos por toda su ayuda y comprensión. A Yandy y a su familia que han estado allí frente a mis dificultades. Agradecer a mi tutor el M.Sc. Ángel Quirós, a mi consultante el Dr.C. José Espinosa y a la Dra.C. María Elena, por su dedicación para el logro de esta tesis. A mis compañeros de grupo por estos cinco años que hemos compartidos juntos, que para mí fueron inolvidables. -
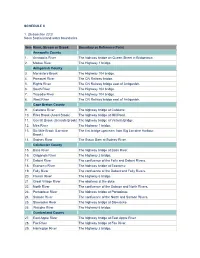
Nova Scotia Inland Water Boundaries Item River, Stream Or Brook
SCHEDULE II 1. (Subsection 2(1)) Nova Scotia inland water boundaries Item River, Stream or Brook Boundary or Reference Point Annapolis County 1. Annapolis River The highway bridge on Queen Street in Bridgetown. 2. Moose River The Highway 1 bridge. Antigonish County 3. Monastery Brook The Highway 104 bridge. 4. Pomquet River The CN Railway bridge. 5. Rights River The CN Railway bridge east of Antigonish. 6. South River The Highway 104 bridge. 7. Tracadie River The Highway 104 bridge. 8. West River The CN Railway bridge east of Antigonish. Cape Breton County 9. Catalone River The highway bridge at Catalone. 10. Fifes Brook (Aconi Brook) The highway bridge at Mill Pond. 11. Gerratt Brook (Gerards Brook) The highway bridge at Victoria Bridge. 12. Mira River The Highway 1 bridge. 13. Six Mile Brook (Lorraine The first bridge upstream from Big Lorraine Harbour. Brook) 14. Sydney River The Sysco Dam at Sydney River. Colchester County 15. Bass River The highway bridge at Bass River. 16. Chiganois River The Highway 2 bridge. 17. Debert River The confluence of the Folly and Debert Rivers. 18. Economy River The highway bridge at Economy. 19. Folly River The confluence of the Debert and Folly Rivers. 20. French River The Highway 6 bridge. 21. Great Village River The aboiteau at the dyke. 22. North River The confluence of the Salmon and North Rivers. 23. Portapique River The highway bridge at Portapique. 24. Salmon River The confluence of the North and Salmon Rivers. 25. Stewiacke River The highway bridge at Stewiacke. 26. Waughs River The Highway 6 bridge. -

Biodiversity Conservation and Habitat Management
CONTENTS BIODIVERSITY CONSERVATION AND HABITAT MANAGEMENT Biodiversity Conservation and Habitat Management - Volume 1 No. of Pages: 458 ISBN: 978-1-905839-20-9 (eBook) ISBN: 978-1-84826-920-0 (Print Volume) Biodiversity Conservation and Habitat Management - Volume 2 No. of Pages: 428 ISBN: 978-1-905839-21-6 (eBook) ISBN: 978-1-84826-921-7 (Print Volume) For more information of e-book and Print Volume(s) order, please click here Or contact : [email protected] ©Encyclopedia of Life Support Systems (EOLSS) BIODIVERSITY CONSERVATION AND HABITAT MANAGEMENT CONTENTS Preface xv VOLUME I Biodiversity Conservation and Habitat Management : An Overview 1 Francesca Gherardi, Dipartimento di Biologia Animale e Genetica, Università di Firenze, Italy Claudia Corti, California Academy of Sciences, San Francisco CA, U.S.A. Manuela Gualtieri, Dipartimento di Scienze Zootecniche, Università di Firenze, Italy 1. Introduction: the amount of biological diversity 2. Diversity in ecosystems 2.1. African wildlife systems 2.2. Australian arid grazing systems 3. Measures of biodiversity 3.1. Species richness 3.2. Shortcuts to monitoring biodiversity: indicators, umbrellas, flagships, keystones, and functional groups 4. Biodiversity loss: the great extinction spasm 5. Causes of biodiversity loss: the “evil quartet” 5.1. Over-harvesting by humans 5.2. Habitat destruction and fragmentation 5.3. Impacts of introduced species 5.4. Chains of extinction 6. Why conserve biodiversity? 7. Conservation biology: the science of scarcity 8. Evaluating the status of a species: extinct until proven extant 9. What is to be done? Conservation options 9.1. Increasing our knowledge 9.2. Restore habitats and manage them 9.3. -

Comparative Anatomy of Four Primitive Muricacean Gastropods: Implications for Trophonine Phylogeny
^/ -S/ COMPARATIVE ANATOMY OF FOUR PRIMITIVE MURICACEAN GASTROPODS: IMPLICATIONS FOR TROPHONINE PHYLOGENY M. G. HARASEWYCH DEPARTMENT OF INVERTEBRATE ZOOLOGY NATIONAL MUSEUM OF NATURAL HISTORY SMITHSONIAN INSTITUTION WASHINGTON, D.C. 20560, U.S.A. ABSTRACT The main features of the shell, head-foot, palliai complex, alimentary and reproductive systems of Trophon geversianus (Pallas), Boreotrophon aculeatus (Watson), Paziella pazi (Crosse), and Nucella lamellosa (Gmelin) are described, and phonetic and cladistic analyses based on subsets of these data presented. Similarities in shell morphology revealed by phenetic studies are interpreted as being due to convergence, and are indicative of similar habitats rather than of close phylogenetic relationships. Convergences are also noted in radular and stomach characters. Cladistic analyses of anatomical data support the following conclusions: 1 ) Thaididae are a primitive and ancient family of muricaceans forming a clade equal in taxonomic rank with Muncidae; 2) Within Muricidae, P. pazi more closely resembles the ancestral muricid phenotype than any trophonine; 3) Trophoninae comprise a comparatively recent monophyletic group with differences due to a subsequent austral adaptive radiation. The Muricidae are considered to be the most primitive and D'Attilio, 1976:13) a personal communication from E. H. family within Neogastropoda according to most (Thiele, Vokes "it appears likely that the most northern trophons are 1929; Wenz, 1941; Taylor and Sohl, 1962; Boss, 1982) but derived from the Paziella-Poiheha line, and that the several not all (Golikov and Starobogatov, 1975) recent classifica- austral forms that are unquestionably "trophonine" are prob- tions. Of the five subfamilies of Muricidae, the Trophoninae, ably derived from the Thaididae". proposed by Cossmann (1903) on the basis of shell and Thus, according to most published work, the Tropho- opercular characters to include a number of boreal and ninae are in a position to shed light on the systematics and austral species, are the most poorly understood. -

Marine Shells of the Western Coast of Flordia
wm :iii! mm ilili ! Sfixing cHdL J^oad .Sandivicl'i, j\{ai.i.ach.u±£.tti. icuxucm \^*^£ FRONTISPIECE Photo by Ruth Bernhard Spondylus americanus Hermann MARINE SHELLS f>4 OF THE WESTERN COAST OF FLORIDA By LOUISE M. PERRY AND JEANNE S. SCHWENGEL With Revisions and Additions to Louise M. Perry's Marine Shells of the Southwest Coast of Florida Illustrations by W. Hammersley Southwick, Axel A. Olsson, and Frank White March, 1955 PALEONTOLOGICAL RESEARCH INSTITUTION ITHACA, NEW YORK U. S. A. MARINE SHELLS OF THE SOUTHWEST COAST OF FLORIDA printed as Bulletins of American Paleontology, vol. 26, No. 95 First printing, 1940 Second printing, 1942 Copyright, 1955, by Paleontological Research Institution Library of Congress Catalog Card Number: 5-^-12005 Printed in the United States of America // is perhaps a more fortunate destiny to have a taste for collecting shells than to be born a millionaire. Robert Louis Stevenson imeters 50 lllllllllllllllllllllllllllll II II III nil 2 Inches CONTENTS Page Preface by reviser 7 Foreword by Wm. J. Clench 9 Introduction 11 Generalia 13 Collection and preparation of specimens 17 Systematic descriptions 24 Class Amphineura :. 24 Class Pelecypoda 27 Class Scaphopoda 97 Class Gasteropoda 101 Plates 199 Index 311 PREFACE BY THE REVISER It has been a privilege to revise Louise M. Perry's fine book on "Marine Shells of Southwest Florida", to include her studies on eggs and larvae of mollusks; and to add descriptions and illustra- tions of several newly discovered shells thus making it a more com- prehensive study of the molluscan life of western Florida. The work that I have done is only a small return to Dr. -

Policy Drafting Template SE-NIS-1 HLMO Living Through
Policy drafting template SE-NIS-1 HLMO Living through Sub bullet(s) Our oceans support environmental limits viable populations of representative, rare, vulnerable, and valued species. Grouping Invasive non-native Code SE-NIS-1 species Policy SE-NIS-1 Proposals that reduce the risk of spread and/or introduction of non-native invasive species within the south east marine plan area and adjacent plan areas should be supported. Proposals must put in place appropriate measures to avoid or minimise significant adverse impacts that would arise through the introduction and transport of non-native invasive species, particularly when: 1) moving equipment, boats or livestock (for example fish or shellfish) from one water body to another 2) introducing structures suitable for settlement of non-native invasive species, or the spread of non-native invasive species known to exist in the area. What are non-native and non-native invasive species? 1. Non-native (sometimes referred to as non-indigenous) species are those introduced outside of their natural past or present distribution which might survive and subsequently reproduce. In many cases non-native species do not cause harm to the local environment or economy. The Great Britain Non-Native Species Secretariat describes non-native invasive species as any non-native species that has the ability to spread, causing damage to the environment, economy, human health or the way we live. Non-native species can become ‘invasive’ when they cause significant adverse impacts. For example, the carpet sea squirt (Didemnum vexillum) was confirmed in British waters in 2008. It spreads quickly and smothers equipment, boat hulls, fishing equipment and native habitats, such as mussel beds.