Comparative Floral Traits in Corunastylis (Diurideae; Orchidaceae) with Novel Applications: Do Some Species Bleed Or Blink?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A New Brachypterous Species of Elachiptera Becker (Diptera: Chloropidae) from Freshwater Wetlands in Eastern Canada
Zootaxa 360: 1–6 (2003) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 360 Copyright © 2003 Magnolia Press ISSN 1175-5334 (online edition) A new brachypterous species of Elachiptera Becker (Diptera: Chloropidae) from freshwater wetlands in eastern Canada TERRY A. WHEELER Department of Natural Resource Sciences, McGill University, Macdonald Campus, Ste-Anne-de-Bellevue, QC, H9X 3V9 CANADA ([email protected]) Abstract Elachiptera aquila sp. nov. is described from freshwater wetlands in Ontario and Quebec, Canada. Its apparent sister species is Elachiptera salinaria Sabrosky and Valley, known from coastal salt marshes in the eastern United States. Although there are Palearctic Elachiptera that are polymor- phic for wing length, this is the first brachypterous species of the genus described from North America. Key Words: Chloropidae, Diptera, Elachiptera, Nearctic, brachypterous, systematics Introduction Elachiptera Becker is one of the more easily recognized genera of Nearctic Chloropidae, primarily because of the broad, strap-like arista that characterizes most included species. Below the generic level, however, many species of Elachiptera are morphologically uni- form. One of the exceptions is Elachiptera salinaria Sabrosky and Valley, a salt marsh species from the eastern United States that can be easily distinguished from other Nearctic Elachiptera species by the broadly rounded occipital margin of the head (Sabrosky and Valley 1987). A new species, apparently closely related to E. salinaria, is described here from freshwater wetland habitats in eastern Canada. This is the first brachypterous species of Nearctic Elachiptera. Materials and Methods Specimens were initially preserved in 70% ethanol and subsequently prepared using a crit- ical-point dryer or chemically dried using hexamethyldisilazane. -

Recovery Plan for the Tallong Midge Orchid (Genoplesium Plumosum)
Approved Recovery Plan Recovery Plan for the Tallong Midge Orchid (Genoplesium plumosum) NSW NATIONAL PARKS AND May 2002 WILDLIFE SERVICE © NSW National Parks and Wildlife Service, 2002. This work is copyright, however material presented in this Plan may be copied for personal use or published for educational purposes, providing that any extracts are fully acknowledged. Apart from this and any other use as permitted under the Copyright Act 1968, no part may be reproduced without prior written permission from NPWS. NSW National Parks and Wildlife Service, 43 Bridge Street, (PO Box 1967) Hurstville NSW 2220 Tel: 02 9585 6444 www.npws.nsw.gov.au Requests for information or comments regarding the recovery program for the Tallong Midge Orchid are best directed to: The Tallong Midge Orchid Recovery Team Coordinator, Threatened Species Unit, NPWS Southern Directorate, PO Box 2115, Queanbeyan NSW 2620 Ph: (02) 6298 9700 Or The Director, Regional Wildlife Programs, Wildlife Australia Branch, Environment Australia, PO Box 636, Canberra ACT 2601 Ph: (02) 6274 1111 Cover illustration: Tallong Midge Orchid. Photographer: John Briggs This Plan should be cited as following: NSW National Parks and Wildlife Service (2002). Approved Recovery Plan for the Tallong Midge Orchid (Genoplesium plumosum). NSW National Parks and Wildlife Service, Hurstville NSW. ISBN 07 313 6457 0 Approved Recovery Plan The Tallong Midge Orchid Recovery Plan for the Tallong Midge Orchid (Genoplesium plumosum) Executive Summary This document constitutes the formal National and New South Wales State Recovery Plan for the Tallong Midge Orchid Genoplesium plumosum. It considers the conservation requirements of the species across its known range, identifies the future actions to be taken to ensure its long-term viability and the parties who will carry these out. -

Vascular Plant and Vertebrate Inventory of Chiricahua National Monument
In Cooperation with the University of Arizona, School of Natural Resources Vascular Plant and Vertebrate Inventory of Chiricahua National Monument Open-File Report 2008-1023 U.S. Department of the Interior U.S. Geological Survey National Park Service This page left intentionally blank. In cooperation with the University of Arizona, School of Natural Resources Vascular Plant and Vertebrate Inventory of Chiricahua National Monument By Brian F. Powell, Cecilia A. Schmidt, William L. Halvorson, and Pamela Anning Open-File Report 2008-1023 U.S. Geological Survey Southwest Biological Science Center Sonoran Desert Research Station University of Arizona U.S. Department of the Interior School of Natural Resources U.S. Geological Survey 125 Biological Sciences East National Park Service Tucson, Arizona 85721 U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark Myers, Director U.S. Geological Survey, Reston, Virginia: 2008 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS-the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web:http://www.usgs.gov Telephone: 1-888-ASK-USGS Suggested Citation Powell, B.F., Schmidt, C.A., Halvorson, W.L., and Anning, Pamela, 2008, Vascular plant and vertebrate inventory of Chiricahua National Monument: U.S. Geological Survey Open-File Report 2008-1023, 104 p. [http://pubs.usgs.gov/of/2008/1023/]. Cover photo: Chiricahua National Monument. Photograph by National Park Service. Note: This report supersedes Schmidt et al. (2005). Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. -

Phylogenetic Relationships of Discyphus Scopulariae
Phytotaxa 173 (2): 127–139 ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ PHYTOTAXA Copyright © 2014 Magnolia Press Article ISSN 1179-3163 (online edition) http://dx.doi.org/10.11646/phytotaxa.173.2.3 Phylogenetic relationships of Discyphus scopulariae (Orchidaceae, Cranichideae) inferred from plastid and nuclear DNA sequences: evidence supporting recognition of a new subtribe, Discyphinae GERARDO A. SALAZAR1, CÁSSIO VAN DEN BERG2 & ALEX POPOVKIN3 1Departamento de Botánica, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado Postal 70-367, 04510 México, Distrito Federal, México; E-mail: [email protected] 2Universidade Estadual de Feira de Santana, Departamento de Ciências Biológicas, Av. Transnordestina s.n., 44036-900, Feira de Santana, Bahia, Brazil 3Fazenda Rio do Negro, Entre Rios, Bahia, Brazil Abstract The monospecific genus Discyphus, previously considered a member of Spiranthinae (Orchidoideae: Cranichideae), displays both vegetative and floral morphological peculiarities that are out of place in that subtribe. These include a single, sessile, cordate leaf that clasps the base of the inflorescence and lies flat on the substrate, petals that are long-decurrent on the column, labellum margins free from sides of the column and a column provided with two separate, cup-shaped stigmatic areas. Because of its morphological uniqueness, the phylogenetic relationships of Discyphus have been considered obscure. In this study, we analyse nucleotide sequences of plastid and nuclear DNA under maximum parsimony -

Otanewainuku ED (Report Prepared on 13 August 2013)
1 NZFRI collection wish list for Otanewainuku ED (Report prepared on 13 August 2013) Fern Ally Isolepis cernua Lycopodiaceae Isolepis inundata Lycopodium fastigiatum Isolepis marginata Lycopodium scariosum Isolepis pottsii Psilotaceae Isolepis prolifera Tmesipteris lanceolata Lepidosperma australe Lepidosperma laterale Gymnosperm Schoenoplectus pungens Cupressaceae Schoenoplectus tabernaemontani Chamaecyparis lawsoniana Schoenus apogon Cupressus macrocarpa Schoenus tendo Pinaceae Uncinia filiformis Pinus contorta Uncinia gracilenta Pinus patula Uncinia rupestris Pinus pinaster Uncinia scabra Pinus ponderosa Hemerocallidaceae Pinus radiata Dianella nigra Pinus strobus Phormium cookianum subsp. hookeri Podocarpaceae Phormium tenax Podocarpus totara var. totara Iridaceae Prumnopitys taxifolia Crocosmia xcrocosmiiflora Libertia grandiflora Monocotyledon Libertia ixioides Agapanthaceae Watsonia bulbillifera Agapanthus praecox Juncaceae Alliaceae Juncus articulatus Allium triquetrum Juncus australis Araceae Juncus conglomeratus Alocasia brisbanensis Juncus distegus Arum italicum Juncus edgariae Lemna minor Juncus effusus var. effusus Zantedeschia aethiopica Juncus sarophorus Arecaceae Juncus tenuis var. tenuis Rhopalostylis sapida Luzula congesta Asparagaceae Luzula multiflora Asparagus aethiopicus Luzula picta var. limosa Asparagus asparagoides Orchidaceae Cordyline australis x banksii Acianthus sinclairii Cordyline banksii x pumilio Aporostylis bifolia Asteliaceae Corunastylis nuda Collospermum microspermum Diplodium alobulum Commelinaceae -

Vascular Plant and Vertebrate Inventory of Fort Bowie National Historic Site Vascular Plant and Vertebrate Inventory of Fort Bowie National Historic Site
Powell, Schmidt, Halvorson In Cooperation with the University of Arizona, School of Natural Resources Vascular Plant and Vertebrate Inventory of Fort Bowie National Historic Site Vascular Plant and Vertebrate Inventory of Fort Bowie National Historic Site Plant and Vertebrate Vascular U.S. Geological Survey Southwest Biological Science Center 2255 N. Gemini Drive Flagstaff, AZ 86001 Open-File Report 2005-1167 Southwest Biological Science Center Open-File Report 2005-1167 February 2007 U.S. Department of the Interior U.S. Geological Survey National Park Service In cooperation with the University of Arizona, School of Natural Resources Vascular Plant and Vertebrate Inventory of Fort Bowie National Historic Site By Brian F. Powell, Cecilia A. Schmidt , and William L. Halvorson Open-File Report 2005-1167 December 2006 USGS Southwest Biological Science Center Sonoran Desert Research Station University of Arizona U.S. Department of the Interior School of Natural Resources U.S. Geological Survey 125 Biological Sciences East National Park Service Tucson, Arizona 85721 U.S. Department of the Interior DIRK KEMPTHORNE, Secretary U.S. Geological Survey Mark Myers, Director U.S. Geological Survey, Reston, Virginia: 2006 For product and ordering information: World Wide Web: http://www.usgs.gov/pubprod Telephone: 1-888-ASK-USGS For more information on the USGS-the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment: World Wide Web:http://www.usgs.gov Telephone: 1-888-ASK-USGS Suggested Citation Powell, B. F, C. A. Schmidt, and W. L. Halvorson. 2006. Vascular Plant and Vertebrate Inventory of Fort Bowie National Historic Site. -

Distribution of Oscinellinae (Diptera: Chloropidae) in the Danish Landscape Lise Brunberg Nielsen
Distribution of Oscinellinae (Diptera: Chloropidae) in the Danish landscape Lise Brunberg Nielsen Nielsen, Lise Brunberg: Distribution of Oscinellinae (Diptera: Chloropidae) in the Danish Landscape. Ent. Meddr 82: 39-62, Copenhagen, Denmark, 2014. ISSN 0013-8851 Abstract About 29,700 Oscinellinae were collected by means of sweep net, water traps and pitfalls in a variety of uncultivated habitats in Denmark mainly in Jutland. So far 75 species belonging to 21 genera are re corded from Denmark. Eleven species are new to the Danish fauna. Morphological details of Aphanotrigonum brachypterum, A. hungaricum, A. nigripes, Conioscinella gallarum, lncertella albipalpis, I. nigrifrons, I. kerteszi, I. scotica and Oscinella angustipennis are presented. The distribu tion of Oscinellinae in the Danish landscape is discussed. In Denmark, farmland dominates, so the two most abundant Oscinellarspecies of ara ble land, Oscinella frit and 0. vastator, are also predominant in most nat ural habitats. Small and larger uncultivated areas, however, making up only 25 % of the Danish landscape, contain a rich fauna of Oscinel lines. The advantage of different sampling methods combined is demonstrated. Sammendrag Fordelingen af fritf1uer (Diptera: Chloropidae) i det danske landskab. De fa millimeter lange, sorte eller sort-gule fritf1uer (Chloropidae) er nogle af de mest almindelige fluer pa gr<esarealer i Danmark. Et start materiale indsamlet med ketcher, i fangbakker og nedgravede fangglas pa forskellige udyrkede gr<esarealer er artsbestemt. Hovedparten af materialet, ea. 29.700 individer tilh0rer underfamilen Oscinellinae, der i Danmark omfatter 21 sl<egter og 75 arter. Elleve arter er nye for den danske fauna. Alle arter er beskrevet i Nartshuk & Andersson (2013), men supplerende morfologiske detaljer er her tilf0jet for 9 af dem: Aphanotrigonum brachypterum, A. -

Prasophyllum Stellatum Stellatum (Ben Lomond Leek-Orchid)
Listing Statement for Prasophyllum stellatum (ben lomond leek-orchid) Prasophyllum stellatum ben lomond leek-orchid T A S M A N I A N T H R E A T E N E D S P E C I E S L I S T I N G S T A T E M E N T Image by Mark Wapstra Scientific name: Prasophyllum stellatum D.L.Jones, Austral. Orchid Res . 3: 115 (1998) Common name: ben lomond leek-orchid (Wapstra et al. 2005) Group: vascular plant, dicotyledon, family Orchidaceae Status: Threatened Species Protection Act 1995 : endangered Environment Protection and Biodiversity Conservation Act 1999 : Critically Endangered Distribution: Endemic status: Endemic to Tasmania Tasmanian NRM Region: North Figure 1 . The distribution of Prasophyllum stellatum Plate 1. Prasophyllum stellatum from the Storys Creek type location (image by Mark Wapstra) 1 Threatened Species Section – Department of Primary Industries, Parks, Water and Environment Listing Statement for Prasophyllum stellatum (ben lomond leek-orchid) IDENTIFICATION AND ECOLOGY withered fertilised flowers) when seen. The Prasophyllum species, commonly known as leek- species may not flower or emerge in dry years. orchids, are deciduous terrestrials with small, fleshy, round or oval tubers and a few fleshy, Description irregular roots. Most species are dormant over The following description for the published summer and autumn and begin growth in early concept of Prasophyllum stellatum is adapted from winter. The single, erect, hollow leaf is reddish Jones (1998), Jones et al. (1999) and Jones at the base as opposed to green as in onion- (2006). However, recent field studies (Wapstra orchids ( Microtis ). -

Flora Vol 3 FC
PLANTS+ OF THE BLACK RANGE OF NEW MEXICO Volume Three Arranged by english common name This checklist recognizes the plant collecting efforts of Anna Isabel Mulford in the Black Range during 1895. PLANTS+ OF THE BLACK RANGE OF NEW MEXICO An Annotated Checklist Edition One of Volume three This checklist of the plants (including a few lichen and other Black Range website, a search for specimen sheets was non-plant species) of the Black Range of southwestern New conducted; Mexico draws from a variety of sources. It is a work in progress and undoubtedly contains errors. If you encounter ✦ If a specimen sheet from the Black Range was located errors of substantive omission or commission or for the species, an entry noting this was made in the administrative errors (broken or incorrect links, spelling, notes column. The name of the collector and the etc.) please let me know at [email protected] so that general location where the specimen was collected the errors can be corrected in the second edition. Your help was entered in the notes column as a link to the in this manner will be of benefit to the general community. specimen sheet. Such entries are shown in dark blue. Methodology ✦ Species which are not verified for the Black Range are indicated by a light blue “cell filling” in the first cell in This checklist was put together in the following manner: the species row. ✦ A search of the SEINet data base (Arizona & New Mexico Chapters) was conducted to determine the Disclaimers and possible species in the Black Range; Points of Clarification ✦ A preliminary search of the Consortium of North In some cases, you may note that an entry from the Vascular American Lichen Herbaria data base was conducted to Plants of the Gila Wilderness data base has been entered on determine possible species in the Black Range (this the checklist but the initial cell of the species listing is filled work is incomplete); in light blue indicating that the species was not verified for the Black Range following the process described above. -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000). -

Redalyc.ARE OUR ORCHIDS SAFE DOWN UNDER?
Lankesteriana International Journal on Orchidology ISSN: 1409-3871 [email protected] Universidad de Costa Rica Costa Rica BACKHOUSE, GARY N. ARE OUR ORCHIDS SAFE DOWN UNDER? A NATIONAL ASSESSMENT OF THREATENED ORCHIDS IN AUSTRALIA Lankesteriana International Journal on Orchidology, vol. 7, núm. 1-2, marzo, 2007, pp. 28- 43 Universidad de Costa Rica Cartago, Costa Rica Available in: http://www.redalyc.org/articulo.oa?id=44339813005 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative LANKESTERIANA 7(1-2): 28-43. 2007. ARE OUR ORCHIDS SAFE DOWN UNDER? A NATIONAL ASSESSMENT OF THREATENED ORCHIDS IN AUSTRALIA GARY N. BACKHOUSE Biodiversity and Ecosystem Services Division, Department of Sustainability and Environment 8 Nicholson Street, East Melbourne, Victoria 3002 Australia [email protected] KEY WORDS:threatened orchids Australia conservation status Introduction Many orchid species are included in this list. This paper examines the listing process for threatened Australia has about 1700 species of orchids, com- orchids in Australia, compares regional and national prising about 1300 named species in about 190 gen- lists of threatened orchids, and provides recommen- era, plus at least 400 undescribed species (Jones dations for improving the process of listing regionally 2006, pers. comm.). About 1400 species (82%) are and nationally threatened orchids. geophytes, almost all deciduous, seasonal species, while 300 species (18%) are evergreen epiphytes Methods and/or lithophytes. At least 95% of this orchid flora is endemic to Australia. -
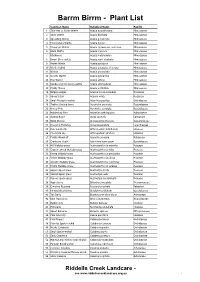
Botanical Name
Barrm Birrm - Plant List Common Name Botanical Name Family 1 Thin-leaf or Snake Wattle Acacia aculeatissima Mimosaceae 2 Silver Wattle Acacia dealbata Mimosaceae 3 Spreading Wattle Acacia genistifolia Mimosaceae 4 Ploughshare Wattle Acacia gunnii Mimosaceae 5 Cinnamon Wattle Acacia leprosa var. uninervia Mimosaceae 6 Black Wattle Acacia mearnsii Mimosaceae 7 Blackwood Acacia melanoxylon Mimosaceae 8 Dwarf Silver-wattle Acacia nano-dealbata Mimosaceae 9 Hedge Wattle Acacia paradoxa Mimosaceae 10 Wattle hybrid Acacia paradoxa x leprosa Mimosaceae 11 Wirilda Acacia provincialis Mimosaceae 12 Golden Wattle Acacia pycnantha Mimosaceae 13 Hop Wattle Acacia stricta Mimosaceae 14 Dandenong Cinnamon-wattle Acacia strictophylla Mimosaceae 15 Prickly Moses Acacia verticillata Mimosaceae 16 Bidgee-widgee Acaena novae-zelandiae Rosaceae 17 Sheep's Burr Acaena ovina Rosaceae 18 Small Mosquito-orchid Acianthus pusillus Orchidaceae 19 Trailing Ground-berry Acrotriche prostrata Epacridaceae 20 Honey Pots Acrotriche serrulata Epacridaceae 21 Maidenhair Fern Adiantum aethiopicum Adiantaceae 22 Austral Bugle Ajuga australis Lamiaceae 23 Black Sheoak Allocasuarina littoralis Casuarinaceae 24 Drooping Mistletoe Amyema pendula Loranthaceae 25 Pale Vanilla-lily Arthropodium milleflorum Liliaceae 26 Chocolate Lily Arthropodium strictum Liliaceae 27 Prickly Woodruff Asperula scoparia Rubiaceae 28 Cranberry Heath Astroloma humifusum Epacridaceae 29 Hill Wallaby-grass Austrodanthonia eriantha Poaceae 30 Copper-awned Wallaby-grass Austrodanthonia fulva Poaceae 31