Article & Appendix
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Chennai Comprehensive Transportation Study (CCTS)
ACKNOWLEDGEMENT The consultants are grateful to Tmt. Susan Mathew, I.A.S., Addl. Chief Secretary to Govt. & Vice-Chairperson, CMDA and Thiru Dayanand Kataria, I.A.S., Member - Secretary, CMDA for the valuable support and encouragement extended to the Study. Our thanks are also due to the former Vice-Chairman, Thiru T.R. Srinivasan, I.A.S., (Retd.) and former Member-Secretary Thiru Md. Nasimuddin, I.A.S. for having given an opportunity to undertake the Chennai Comprehensive Transportation Study. The consultants also thank Thiru.Vikram Kapur, I.A.S. for the guidance and encouragement given in taking the Study forward. We place our record of sincere gratitude to the Project Management Unit of TNUDP-III in CMDA, comprising Thiru K. Kumar, Chief Planner, Thiru M. Sivashanmugam, Senior Planner, & Tmt. R. Meena, Assistant Planner for their unstinted and valuable contribution throughout the assignment. We thank Thiru C. Palanivelu, Member-Chief Planner for the guidance and support extended. The comments and suggestions of the World Bank on the stage reports are duly acknowledged. The consultants are thankful to the Steering Committee comprising the Secretaries to Govt., and Heads of Departments concerned with urban transport, chaired by Vice- Chairperson, CMDA and the Technical Committee chaired by the Chief Planner, CMDA and represented by Department of Highways, Southern Railways, Metropolitan Transport Corporation, Chennai Municipal Corporation, Chennai Port Trust, Chennai Traffic Police, Chennai Sub-urban Police, Commissionerate of Municipal Administration, IIT-Madras and the representatives of NGOs. The consultants place on record the support and cooperation extended by the officers and staff of CMDA and various project implementing organizations and the residents of Chennai, without whom the study would not have been successful. -
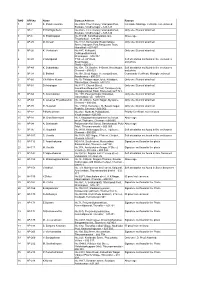
SNO APP.No Name Contact Address Reason 1 AP-1 K
SNO APP.No Name Contact Address Reason 1 AP-1 K. Pandeeswaran No.2/545, Then Colony, Vilampatti Post, Intercaste Marriage certificate not enclosed Sivakasi, Virudhunagar – 626 124 2 AP-2 P. Karthigai Selvi No.2/545, Then Colony, Vilampatti Post, Only one ID proof attached. Sivakasi, Virudhunagar – 626 124 3 AP-8 N. Esakkiappan No.37/45E, Nandhagopalapuram, Above age Thoothukudi – 628 002. 4 AP-25 M. Dinesh No.4/133, Kothamalai Road,Vadaku Only one ID proof attached. Street,Vadugam Post,Rasipuram Taluk, Namakkal – 637 407. 5 AP-26 K. Venkatesh No.4/47, Kettupatti, Only one ID proof attached. Dokkupodhanahalli, Dharmapuri – 636 807. 6 AP-28 P. Manipandi 1stStreet, 24thWard, Self attestation not found in the enclosures Sivaji Nagar, and photo Theni – 625 531. 7 AP-49 K. Sobanbabu No.10/4, T.K.Garden, 3rdStreet, Korukkupet, Self attestation not found in the enclosures Chennai – 600 021. and photo 8 AP-58 S. Barkavi No.168, Sivaji Nagar, Veerampattinam, Community Certificate Wrongly enclosed Pondicherry – 605 007. 9 AP-60 V.A.Kishor Kumar No.19, Thilagar nagar, Ist st, Kaladipet, Only one ID proof attached. Thiruvottiyur, Chennai -600 019 10 AP-61 D.Anbalagan No.8/171, Church Street, Only one ID proof attached. Komathimuthupuram Post, Panaiyoor(via) Changarankovil Taluk, Tirunelveli, 627 761. 11 AP-64 S. Arun kannan No. 15D, Poonga Nagar, Kaladipet, Only one ID proof attached. Thiruvottiyur, Ch – 600 019 12 AP-69 K. Lavanya Priyadharshini No, 35, A Block, Nochi Nagar, Mylapore, Only one ID proof attached. Chennai – 600 004 13 AP-70 G. -

Summary of Family Membership and Gender by Club MBR0018 As of February, 2010
Summary of Family Membership and Gender by Club MBR0018 as of February, 2010 Club Fam. Unit Fam. Unit Club Ttl. Club Ttl. District Number Club Name HH's 1/2 Dues Females Male TOTAL District 324A6 26412 AVADI 5 5 6 49 55 District 324A6 26418 ENNORE TIRUVOTTIYUR 0 0 2 8 10 District 324A6 26438 CHENNAI SHANTHI COLONY 3 3 3 11 14 District 324A6 26449 TAMBARAM 1 1 0 19 19 District 324A6 29705 VILLAVAKKAM 3 3 4 18 22 District 324A6 32179 PALLAVAPURAM 6 6 6 39 45 District 324A6 32675 TAMBARAM WEST 1 0 5 27 32 District 324A6 35460 MADRAS METROPOLITAN SO 5 5 6 12 18 District 324A6 36531 MADRAS RED HILLS 25 26 27 90 117 District 324A6 37537 MADRAS METRO EAST 48 48 55 80 135 District 324A6 40288 MADRAS SEMBIUM 2 2 2 21 23 District 324A6 45544 MADRAS SELAIYUR 0 0 0 18 18 District 324A6 49225 MADRAS TRIDENT PALLAVARAM 0 0 0 19 19 District 324A6 51650 MADRAS PERIPHERAL CITY 8 9 7 33 40 District 324A6 54882 MADRAS ARUMBAKKAM 0 0 0 12 12 District 324A6 55709 MADRAS KOLATHOOR 0 0 0 8 8 District 324A6 57011 CHITLAPAKKAM 0 0 1 39 40 District 324A6 60194 MADRAS HASTHINAPURAM 4 4 6 11 17 District 324A6 60920 CHENNAI AMBASSADORS 11 11 11 22 33 District 324A6 61600 MADRAS MADIPAKKAM 0 0 0 16 16 District 324A6 62049 MADRAS BHARATHAM-PERAVALLUR 0 0 1 15 16 District 324A6 62244 CHENNAI TIRUVOTTIYUR EAST 1 1 1 23 24 District 324A6 62543 MADRAS TAMBARAM EAST 4 5 9 16 25 District 324A6 63671 CHENNAI MADAMBAKKAM 0 0 0 23 23 District 324A6 63681 CHENNAI AGARAM 0 0 0 26 26 District 324A6 63701 CHENNAI GUMMIDIPOONDI 1 1 1 30 31 District 324A6 64330 CHENNAI KUNRATHUR -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2012 [Price: Rs. 28.80 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 37] CHENNAI, WEDNESDAY, SEPTEMBER 19, 2012 Purattasi 3, Thiruvalluvar Aandu–2043 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Change of Names .. 2325-2395 Notices .. 2240-2242 .. 1764 1541-1617 NoticeNOTICE .. 1617 NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES 34603. My daughter, N. Rizwana, born on 12th November 34606. I, B. Vel, son of Thiru K. Bethuraj, born on 4th April 1996 (native district: Ramanathapuram), residing at 1967 (native district: Theni), residing at No. 63-NA, Vaigai No. 11-7B/68, New Ramnad Road, Visalam, Madurai- Colony, Anna Nagar, Madurai-625 020, shall henceforth be 625 009, shall henceforth be known as N. RISVANA BEGAM. known as B. VELUCHAMY. ï£Ã˜H„¬ê. B. VEL. Madurai, 10th September 2012. (Father.) Madurai, 10th September 2012. 34604. I, N. Radha, daughter of Thiru V. Navaneethan, 34607. I, N. Thirupathi, son of Thiru Narayanasamy, born born on 18th July 1991 (native district: Calcutta-West Bengal), on 7th June 1983 (native district: Dindigul), residing at residing at No. 1/65A, Katchaikatti, T. Vadipatti Taluk, Madurai-625 218, shall henceforth be known No. 1C, Melaponnakaram 2nd Street, Arapalayam Post, as N. -

Storm Water Flood Modeling in the Sub- Basin of Chennai Corporation, Chennai, Tamilnadu, India
Storm Water Flood Modeling in the Sub- basin of Chennai Corporation, Chennai, Tamilnadu, India Dr. Y. R. Satyaji Rao Scientist F and Head Deltaic Regional Centre National Institute of Hydrology Kakinada 533003, Andhra Pradesh, India Website: www.nih.ernet.in Types of Flooding (Geographically): Riverine flooding It happens when extreme rainfall attacks in a river basin (Mississippi, 1993; Miller, 1997; Changman, 1998; Li and Guo et al., 1999; NVE, 2000; Meade, 2002). Urban flooding It is triggered when surface runoff exceeds the capacity of drainage systems, which happens when heavy rainfall pours on sewers with the limited capacity, or even medium rainfall falls on poorly planned or operated drainage systems (Kamal and Rabbi, 1998; Arambepola, 2002). Coastal flooding It takes place when heavy rainfall on inland encounters storm surges from the sea (Miller, 1997; Barry, 1997; Smith and Ward, 1998; Parker1, 2000; Pilarczyk and Nuoi, 2002). Definition of Urban Drainage Systems Urban drainage systems are defined as physical facilities that collect, store, convey, and treat runoff in urban areas. These facilities normally include detention and retention facilities, streets, storm sewers, inlets, open channels, and special structures such as inlets, manholes, and energy dissipaters” (ASCE and WEF, 1992). Why Urban Floods are increasing ? Increase in Flood peak and storm water network designs are old (Design limitations) Improper maintenance of storm water network (Carrying capacity) Impact of Boundary Conditions (Confluence points/backwater/tides) -

B29N Bus Time Schedule & Line Route
B29N bus time schedule & line map B29N Otteri View In Website Mode The B29N bus line (Otteri) has 4 routes. For regular weekdays, their operation hours are: (1) Otteri: 8:55 PM (2) Perambur B.S.: 10:00 AM (3) Periyar Nagar: 6:58 PM (4) Velachery: 8:10 AM - 5:28 PM Use the Moovit App to ƒnd the closest B29N bus station near you and ƒnd out when is the next B29N bus arriving. Direction: Otteri B29N bus Time Schedule 16 stops Otteri Route Timetable: VIEW LINE SCHEDULE Sunday 8:55 PM Monday 8:55 PM Periyar Nagar Tuesday 8:55 PM S.R.B Colony Wednesday 8:55 PM 70 Feet Road Thursday 8:55 PM Agaram Junction Friday 8:55 PM Kamarajar Salai Saturday 8:55 PM Veenus Chembium Old Post O∆ce B29N bus Info Lourde School Direction: Otteri Stops: 16 Trip Duration: 11 min Mangalapuri Line Summary: Periyar Nagar, S.R.B Colony, 70 Feet Chandra Yogi Samadhi Rd, Chennai Road, Agaram Junction, Kamarajar Salai, Veenus, Chembium Old Post O∆ce, Lourde School, Perambur Mangalapuri, Perambur, Jamalaya, Mettupalayam, Otteri Church, Binny Mills, Otteri Police Station, Otteri Jamalaya Mettupalayam Otteri Church Binny Mills Otteri Police Station Otteri Kancheepuram, Chennai Otteri Otteri Bridge, Chennai Direction: Perambur B.S. B29N bus Time Schedule 56 stops Perambur B.S. Route Timetable: VIEW LINE SCHEDULE Sunday 10:00 AM Monday 10:00 AM Velachery (Vijaynagar) Velachery Bypass Road, Chennai Tuesday 10:00 AM Velachery (Vijaynagar) Wednesday 10:00 AM Bus Way, Chennai Thursday 10:00 AM Dhandeswaram Friday 10:00 AM Thandeeswaram Saturday 10:00 AM Gandhi Nagar Gurunanak College Velachery Main Road, Chennai B29N bus Info Direction: Perambur B.S. -

Macro Drainage System in Cma
Chapter X MACRO DRAINAGE SYSTEM IN CMA Introduction: CMA lies along the east coast of Southern India and is traversed by three major rivers namely Kosasthalaiyar River, Cooum River and Adyar River. The climate of the region is dominated by the monsoons, which are caused by thermal contrast between land and sea. Monsoon climates are characterised by clearly marked seasons with specific types of wind and weather. The South West monsoon dominates weather patterns in Tamilnadu from July –September and is characterised by periods of sultry wet weather. Rain shadow effects limit rainfall in the east coast in Tamilnadu and it is light or intermittent during this season. This period is followed by North-East Monsoon, which brings cool cloudy weather, relatively free of rain over most of the monsoon- dominated land (India). The exception is South-East-India including Tamilnadu where about 78% of the annual rainfall occurs at this time. The start of the heavy rains usually falls in October lasting up to December. Most of the rainfall is associated with clear synoptic systems of depressions and cyclones with night time rainfall most common. In CMA between October and December most of the rainfall occurs and it is rare between January and April. 10.02 River Nagari which has a large catchment area in the Chittoor District (Andhra Pradesh) region and the Nandi River, which has catchment area in the Vellore District, join near Kanakamma Chattiram and enter Poondi Reservoir. Kosasthalaiyar River, which has its origin near Kaveripakkam and has catchment area in North Arcot District, has a branch near Kesavaram Anicut and flows to the city as Cooum River and the main Kosasthalaiyar river flows to Poondi reservoir. -

Mint Building S.O Chennai TAMIL NADU
pincode officename districtname statename 600001 Flower Bazaar S.O Chennai TAMIL NADU 600001 Chennai G.P.O. Chennai TAMIL NADU 600001 Govt Stanley Hospital S.O Chennai TAMIL NADU 600001 Mannady S.O (Chennai) Chennai TAMIL NADU 600001 Mint Building S.O Chennai TAMIL NADU 600001 Sowcarpet S.O Chennai TAMIL NADU 600002 Anna Road H.O Chennai TAMIL NADU 600002 Chintadripet S.O Chennai TAMIL NADU 600002 Madras Electricity System S.O Chennai TAMIL NADU 600003 Park Town H.O Chennai TAMIL NADU 600003 Edapalayam S.O Chennai TAMIL NADU 600003 Madras Medical College S.O Chennai TAMIL NADU 600003 Ripon Buildings S.O Chennai TAMIL NADU 600004 Mandaveli S.O Chennai TAMIL NADU 600004 Vivekananda College Madras S.O Chennai TAMIL NADU 600004 Mylapore H.O Chennai TAMIL NADU 600005 Tiruvallikkeni S.O Chennai TAMIL NADU 600005 Chepauk S.O Chennai TAMIL NADU 600005 Madras University S.O Chennai TAMIL NADU 600005 Parthasarathy Koil S.O Chennai TAMIL NADU 600006 Greams Road S.O Chennai TAMIL NADU 600006 DPI S.O Chennai TAMIL NADU 600006 Shastri Bhavan S.O Chennai TAMIL NADU 600006 Teynampet West S.O Chennai TAMIL NADU 600007 Vepery S.O Chennai TAMIL NADU 600008 Ethiraj Salai S.O Chennai TAMIL NADU 600008 Egmore S.O Chennai TAMIL NADU 600008 Egmore ND S.O Chennai TAMIL NADU 600009 Fort St George S.O Chennai TAMIL NADU 600010 Kilpauk S.O Chennai TAMIL NADU 600010 Kilpauk Medical College S.O Chennai TAMIL NADU 600011 Perambur S.O Chennai TAMIL NADU 600011 Perambur North S.O Chennai TAMIL NADU 600011 Sembiam S.O Chennai TAMIL NADU 600012 Perambur Barracks S.O Chennai -

School-Phone-Nos
SCHL_NAME SCHOOL ADDRESS PHONE HM MOBILE 89, 2ND MAIN ROAD, JAGANATHAM NAGAR GOVT HS VILLIWAKKAM CH 49 SOUTH, VILLIVAKKAM, CHENNAI 26170816V.MURUGESAN 9381234553 GOVT HSS GKMCOLONY CHENNAI 82 GKM COLONY, CHENNAI 25509881T RAJAGOPAL 9841052090 GOVT HS BV COLONY VYASARPADI BV COLONY , VYASARPADI, CHENNAI 25513210 JEBAKUMARI BREMAHGNANAKILI9840266547 5TH BLOCK, KKD NAGAR, KODUNGAIYUR, GOVT HS KODUNGAIYUR CH 118 CHENNAI 25544994 R.GOPALAKRISHNAN 9841836254 GOVT HSS MKB NAGAR VYASARPADI MKB NAGAR, VYASARPADI,CHENNAI 26733710 K SEKAR 9444766336 GOVT GIRLS HSS VILLIVAKKAM SIDCONAGAR, VILLIVAKKAM, CHENNAI 26172919R BOMMAKKAL 9444312767 CHENNAI HS STRAHANS ROAD CH 12 4 STRAHANS ROAD, PATTALAM,CHENNAI 26625538 K NAGALAKSHMI 9941816876 CHENNAI HSS BUNDERGARDEN CH-11 NO 38 BUNDER GARDEN ST, CHENNAI 25583683M MURUGAIYAN 9840493327 35,CHINNA BABU STREET, NAMMALWARPET, CHENNAI HS NAMMALWARPET CH- 26472750MUNIKUMARI 9840956058 CHENNAI HSS AYANAVARAM CH 23 NO 1 PALAVOYAL STREET, AYANAVARAM 26742672C KALAI SELVI 7305860299 CHENNAI HS N P KOIL ST CH-1 N.P.KOIL STREET, CHENNAI 25588123SHEEBA .S. 8148877295 CHENNAI G HS CHENNAI 12 26, COOKS ROAD, CHENNAI - 12 26622375 T.PADMAVATHI 9840518909 53, MARKET STREET, PERAMBUR, CHENNAI - CHENNAI GHSS MARKET ST CHENNAI 11 26703477ALAMELU 9176322667 CHENNAI HS WALTAXRD CHENNAI 79 61, WALLTAX ROAD, CHENNAI 25296245R.RAJAKUMARI 9789044906 CHENNAI HS AGARAM CH 82 SOMAIAH RAJA STREET, AGARAM, CHENNAI 26703198S.CHANDAR 9444954941 29-A, GANGADEESWARAR KOIL ST, PURASAI, CHENNAI B HSS GK ST CHENNAI 84 CH-7 26403388 SHEILA ROSE -

Chennai District Tamil Nadu
For official use Technical Report Series DISTRICT GROUNDWATER BROCHURE CHENNAI DISTRICT TAMIL NADU By T. Balakrishnan, Scientist-D Government of India Ministry of Water Resources Central Ground Water Board South Eastern Coastal Region Chennai November 2008 DISTRICT AT A GLANCE (CHENNAI DISTRICT) S. No. ITEMS STATISTICS 1. GENERAL INFORMATION i. Geographical area (Sq. km) 174 ii. Administrative Divisions (As on 31-3-2007) Number of Taluks 5 Corporation 1 iii. Population (As on 2001 Census) Total Population 4343645 Male 2219539 Female 2124106 iv. Average Annual Rainfall (mm) 1200 2. GEOMORPHOLOGY i. Major physiographic Units 1. Fluvial land forms 2. Marine land forms 3. Erosional land forms ii. Major Drainages . 3. LAND USE (Sq. km) (2005-06) Adyar & Cooum i. Forest area 3 ii. Net area sown - iii. Cultivable area - 4. MAJOR SOIL TYPES Beach sands, Clay & alluvial soils 5. NUMBER OF GROUND WATER MONITORING WELLS OF CGWB (As on 01.05.2008) i. Dug wells 11 ii. Piezometers 3 6. PREDOMINANT GEOLOGICAL Alluvium, sandstones FORMATIONS (argillaceous), clay, shale, silt stone, granites, gneisses and charnockite 7. HYDROGEOLOGY i. Major water bearing formations Sand, sandstone, weathered and fractured granites, gneisses and Charnockite ii. Pre monsoon depth to water level (May 2006) 2.21–7.64 m bgl iii. Post monsoon depth to water level (Jan. 2007) 0.45-5.32 m bgl iv. Long term water level trend in 10 years (1998- Annual 2007) (m/yr) Rise Fall Min: 0.003 Min:0. 04 Max: 0.93 Max:0. 78 i 8. GROUND WATER EXPLORATION BY CGWB (As on 31-03-2007) i. -

29B Bus Time Schedule & Line Route
29B bus time schedule & line map 29B Perambur B.S - Saidapet View In Website Mode The 29B bus line (Perambur B.S - Saidapet) has 2 routes. For regular weekdays, their operation hours are: (1) Perambur B.S.: 6:17 AM - 8:10 PM (2) Saidapet: 5:15 AM - 6:45 PM Use the Moovit App to ƒnd the closest 29B bus station near you and ƒnd out when is the next 29B bus arriving. Direction: Perambur B.S. 29B bus Time Schedule 29 stops Perambur B.S. Route Timetable: VIEW LINE SCHEDULE Sunday 6:17 AM - 8:10 PM Monday 6:17 AM - 8:10 PM Saidapet Tuesday 6:17 AM - 8:10 PM Thadandar Nagar Wednesday 6:17 AM - 8:10 PM Cit Nagar Thursday 6:17 AM - 8:10 PM Thiagaraya Nagar Friday 6:17 AM - 8:10 PM Panagal Park Saturday 6:17 AM - 8:10 PM Pondy Bazaar Raja Badar Street, Chennai Vanavali 29B bus Info Direction: Perambur B.S. Teynampet Stops: 29 Trip Duration: 35 min Anna Salai Bus Lane, Chennai Line Summary: Saidapet, Thadandar Nagar, Cit D.M.S.Metro Station Nagar, Thiagaraya Nagar, Panagal Park, Pondy Bazaar, Vanavali, Teynampet, D.M.S.Metro Station, Brs Hospital, Valluvar Kottam, Corporation Brs Hospital School/O∆ce, Sterling Market, Sterling Road, Chetpet, Kmc, Neyveli House, Purasawalkkam, Valluvar Kottam Doveton, Vasanthi Theatre, Kutty Street, Pattalam, Dasamahan, Dasamahan, Otteri, Binny Mill Canteen, Corporation School/O∆ce Otteri Church, Jamalaya, Perambur Sterling Market Sterling Road Chetpet Kmc Neyveli House EVR Periyar Salai (Poonamallee High Road), Chennai Purasawalkkam Doveton Doveton Flyover, Chennai Vasanthi Theatre Kutty Street Pattalam Dasamahan -

2018-2019 Priyadharshini.G No.55 Bharathi Nagar 2Nd Street
5.2.1 Average percentage of placement of outgoing students during the last five years (10) Name of student placed and Program graduated Name of the employer with Pay package at Year contact details from contact details appointment 2018-2019 (Total 121) Priyadharshini.G DHANA CHIT FUNDS No.55 Bharathi Nagar 2nd Street, 2018-2019 B.Com General No:105/223,Triplicane High 1,80,000 Per annum villivakkam, Chennai 600049, Road,Triplicane ,Chennai -05 7358610160 Prashanth.N, DHANA CHIT FUNDS No 82/2 Rajamangalam 3rd 2018-2019 B.Com General No:105/223,Triplicane High 1,80,000 Per annum Street, villivakkam Chennai Road,Triplicane ,Chennai -06 600049,9042870239 Saravanan. P BEREZIA TECHNOLOGY No. 98, Gangai Amman koil 2,Gokulam Colony, Ganesh 2018-2019 B.Com General 1,44,000 Per annum street MGR Nagar, kolathur, Nagar, Guindy, Chennai - Chennai 600099 600042 Mukesh. M BEREZIA TECHNOLOGY No 2/153,3rd main road, 2,Gokulam Colony, Ganesh 2018-2019 Bharathidasan Nagar, B.Com General 1,44,000 Per annum Nagar, Guindy,Chennai - shanmugapuram, Redhills Road, 600043 Chennai 600099, 9952026925 CHRISTINA FINANCE & Muthulakshmi.K INVESTMENT LTD No 343, 8th Street, Saraswathi 2018-2019 B.Com General No: 11,NRD Towers, 1,20,000 Per annum Nagar, Thirumullaivoyal, Jawaharlal Nehru Road, Chennai 600062, 8939665510 Ashok Nagar, Chennai- 80 CHRISTINA FINANCE & Ramya. R INVESTMENT LTD No 117, Elango Street, MKB 2018-2019 B.Com General No: 11,NRD Towers, 1,20,000 Per annum Nagar, Ambattur, Chennai Jawaharlal Nehru Road, 600053, 9791155281 Ashok Nagar, Chennai- 81 SVS INFOTECH Kowsalya. J Mr.Vadivel.P No.