Molecular Population Divergence and Sexual Selection on Morphology in the Banded Demoiselle (Calopteryx Splendens)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Dragonfly News 66
Dragonfly News 66 The Magazine of the British Dragonfly Society Autumn 2014 www.british-dragonflies.org.uk Meet the new BDS Chairman, How many Willow Emeralds are David Chelmick ovipositing? Dragonfly hunting....in Sweden? Andy Holt’s unique larval portraits How tatty can a dragonfly be and still fly? Dragonfly News 66 The Magazine of the British Dragonfly Society Published twice a year, in April and October, Dragonfly News covers all aspects of the British Dragonfly Society’s field, recording, monitoring, research, conservation and social activities, as well as information from the wider dragonfly, natural history and conservation world. The emphasis is on dragonflies recorded in the UK. The British Dragonfly Society aims to promote and encourage the study, conservation and understanding of dragonflies and their natural habitats, especially in the UK, and to raise public awareness of dragonflies. Dragonfly News is edited & designed by: Trustees & Officers of the BDS Mark Tyrrell, 8 Warwick Close, Raunds, Chairman: David Chelmick Northants., NN9 6JH Tel. Vice-Chairman: Vacant e-mail: Secretary: Henry Curry, 23 Bowker Way, Whittlesey, Peterborough, PE7 1PY. Tel. Deadlines for inclusion of copy: Spring 31 January Treasurer: Brian Walker, 49 Roman Way, Wantage, Autumn 31 July Oxfordshire, OX12 9YF. Tel. Advertising Rates: Trustees: David Goddard, Stuart Irons, Mick Parfitt. £15 for small-ad (text only); £40 for quarter- Journal Editor: Peter Mill, 8 Cookridge Grove, LEEDS, page; £60 for half-page; £100 for full-page. LS16 7LH. Shop Manager: Lynn Curry, 23 Bowker Way, Whittlesey, Peterborough, PE7 1PY Tel. © British Dragonfly Society 2014 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any Dragonfly Conservation Group (DCG) form or by any means, electronic, mechanical, photocopying, Convenor: Dave Smallshire, 8, Twindle Beer, Chudleigh, Newton recording or otherwise, without the permission of the British Abbot, Devon, TQ13 0JP. -

175 Post-Copulatory Behaviour in Calopteryx
International Journal of Odonatology 1 (2): 175-184, 1998. © 1998 Backhuys Publishers. 175 POST-COPULATORY BEHAVIOUR IN CALOPTERYX FEMALES (INSECTA, ODONATA, CALOPTERYGIDAE). Martin Lindeboom Institut fiir systematische Zoologie, Eberhard-Karls-Universitiit Ttibingen, Auf der Morgenstelle 28, 72076 Ttibingen, Germany, e-mail: [email protected] Received 30 May 1998; revised 12 October 1998; accepted 13 October 1998. Key words: Odonata, Calopteryx, post-copulatory behaviour, functional morphology, ovipositor, copulatory organ, microstructures, sperm removal, cryptic female choice. Abstract The post-copulatory behaviour of Calopteryx splendens females was studied under field and laboratory conditions. After termination of copulation females usually perch and bend the abdomen so that its apex touches the ground (post-copulatory posture). The post-cop ulatory posture is a consequence of sperm removal by males. Male and female microstruc tures (spines and scales) interact to move previous sperm from the female sperm storage organs to the outside during copulation stage I, after which moved sperm is located on the ovipositor. After termination of copulation females require an average of 45 seconds to brush off this sperm (N=21). The post-copulatory behaviour of females may also allow males to chase rival males before the females start to oviposit (prevention of dis turbances). The present study shows no evidence of cryptic female choice in C. splendens. Introduction Sperm competition in Odonata has resulted in the evolution of direct sperm removal in Zygoptera and Anisoptera. Sperm removal resulting in last male sperm precedence seems to be widespread in Zygoptera (Waage 1979a, 1984, 1986; Miller & Miller 1981; Fincke 1984; Siva-Jothy & Tsubaki 1989; Cordero & Miller 1992; Siva-Jothy & Hooper 1995). -

Index to Contents
Index to Contents Author(s) Title Year Vol Pages Holland, Sonia Dragonfly Survey Reports – 1. Gloucestershire 1983 1 (1) 1-3 Butler, Stephen Notes on finding larvae of Somatochlora arctica (Zetterstedt) in N. W. Scotland 1983 1 (1) 4-5 Winsland, David Some observations on Erythromma najas (Hansemann) 1983 1 (1) 6 Merritt, R. Is Sympetrum nigrescens Lucas a good species? 1983 1 (1) 7-8 Vick, G. S. Is Sympetrum nigrescens Lucas a good species? 1983 1 (1) 7-8 Merritt, R. Coenagrion mercuriale (Charpentier) with notes on habitat 1983 1 (1) 9-12 Chelmick, D. G. Observations on the ecology and distribution of Oxygastra curtisii (Dale) 1983 1 (2) 11-14 Khan, R. J. Observations of Wood-mice (Apodemus sylvaticus) and Hobby (Falco subbuteo) feeding on dragonflies 1983 1 (2) 15 Marren, P. R. Scarce Species Status Report 2. A review of Coenagrion hastulatum (Charpentier) in Britain 1983 1 (2) 16-19 Merritt, R. Is Sympetrum nigrescens Lucas a good species? 1983 1 (2) 16-19 Mayo, M. C. A. Coenagrion mercuriale (Charpentier) on the flood plains of the River Itchen and River Test in Hampshire 1983 1 (2) 20-21 Welstead, A. R. Coenagrion mercuriale (Charpentier) on the flood plains of the River Itchen and river Test in Hampshire 1983 1 (2) 20-21 Kemp, R. G. Notes and observations on Gomphus vulgatissimus (Linnaeus) on the river Severn and River Thames 1983 1 (2) 22-25 Vick, G. S. Notes and observations on Gomphus vulgatissimus (Linnaeus) on the river Severn and River Thames 1983 1 (2) 22-25 Corbet, P. -

Diversity of Predatory Arthropods in Bt and Non-Bt Cotton Fields Of
Journal of Entomology and Zoology Studies 2021; 9(2): 1199-1203 E-ISSN: 2320-7078 P-ISSN: 2349-6800 Diversity of predatory arthropods in Bt and non- www.entomoljournal.com JEZS 2021; 9(2): 1199-1203 Bt cotton fields of Nalgonda district, Telangana © 2021 JEZS Received: 22-01-2021 state, India Accepted: 24-02-2021 Modala Mallesh Environmental Biology Lab, Modala Mallesh and Ch. Sravanthy Department of Zoology, Kakatiya University, Warangal, Telangana, India Abstract The study was conducted to investigate diversity of predatory arthropods (Predatory insects and Spiders) Ch. Sravanthy Environmental Biology Lab, in Bt cotton and non-Bt cotton fields during July 2018 to January 2019 at farmers field of Palem Village, Department of Zoology, Kakatiya Nalgonda District, T.S, India. A total 4768 individual arthropods on Bt cotton, 5232 individual University, Warangal, Telangana, arthropods on non-Bt cotton fields were collected with the help of sweep net and hand picking. The India predatory arthropods were identified with the help of Guide on cotton pests and predators, Regional Agricultural Research Station PJTSAU Warangal and literature. Eleven predatory insects and two spiders in Bt cotton and thirteen predatory insects and three spiders in non-Bt cotton were recorded. The results indicated that minor differences found between the Bt and non-Bt cotton fields. Our findings conclude that Bt cotton may affect predatory arthropods indirectly through removal of eggs, larvae and pupa of insect pests that serve as food for predatory arthropods. Various diversity indices were measured. Keywords: Bt cotton, non-Bt cotton, predatory arthropods, ecological indexes and seasonal abundance Introduction Cotton, Gossypium hirsutum L., belonging to Family Malvaceae; Order Malvales is one of the most important commercial crop, playing a key role in economic, political and social affairs of the world. -

© 2016 David Paul Moskowitz ALL RIGHTS RESERVED
© 2016 David Paul Moskowitz ALL RIGHTS RESERVED THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY By DAVID P. MOSKOWITZ A dissertation submitted to the Graduate School-New Brunswick Rutgers, The State University of New Jersey In partial fulfillment of the requirements For the degree of Doctor of Philosophy Graduate Program in Entomology Written under the direction of Dr. Michael L. May And approved by _____________________________________ _____________________________________ _____________________________________ _____________________________________ New Brunswick, New Jersey January, 2016 ABSTRACT OF THE DISSERTATION THE LIFE HISTORY, BEHAVIOR AND CONSERVATION OF THE TIGER SPIKETAIL DRAGONFLY (CORDULEGASTER ERRONEA HAGEN) IN NEW JERSEY by DAVID PAUL MOSKOWITZ Dissertation Director: Dr. Michael L. May This dissertation explores the life history and behavior of the Tiger Spiketail dragonfly (Cordulegaster erronea Hagen) and provides recommendations for the conservation of the species. Like most species in the genus Cordulegaster and the family Cordulegastridae, the Tiger Spiketail is geographically restricted, patchily distributed with its range, and a habitat specialist in habitats susceptible to disturbance. Most Cordulegastridae species are also of conservation concern and the Tiger Spiketail is no exception. However, many aspects of the life history of the Tiger Spiketail and many other Cordulegastridae are poorly understood, complicating conservation strategies. In this dissertation, I report the results of my research on the Tiger Spiketail in New Jersey. The research to investigate life history and behavior included: larval and exuvial sampling; radio- telemetry studies; marking-resighting studies; habitat analyses; observations of ovipositing females and patrolling males, and the presentation of models and insects to patrolling males. -

Report on a Survey of Dragonflies and Damselflies on the River Kird 2017
Report on a survey of Dragonflies and Damselflies on the River Kird 2017 By Dr Alison Barker F.R.E.S. April 2018 Newly emerged Scarce Chaser Dragonfly on the River Kird in May © A.M. Barker 1 Contents Summary Page 3 Introduction Page 3 Introduction to British Dragonflies and Damselflies Page 3 Survey scope and methodology Page 5 Potential limitations Page 7 Water testing Page 7 Site descriptions Page 8 Map (Figure 9) Page 9 Results Page 19 Species recorded Page 19 Site-by-site observations Page 20 Analysis and Discussion Page 30 The Lower Kird as a habitat for Damselflies and Dragonflies. Page 30 Odonata of the River Kird in the context of the Upper Arun S.S.S.I. Page 34 Water quality and Odonata on the River Kird Page 36 Conservation of the River Kird for Dragonflies and Damselflies- Page 37 Trees Page 37 Vegetation and bank structure Page 38 Management of the surrounding habitat Page 39 River habitat diversity and river management Page 39 Invasive species Page 40 Conclusions Page 40 Acknowledgements Page 41 Glossary Page 41 References Page 43 Appendix 1 Table showing all species recorded by site and visit page 46 2 Summary The River Kird is a feeder stream for the River Arun in the Upper Arun Site of Special Scientific Interest (S.S.S.I.) area. Although the latter is designated principally on the richness of its dragonfly and damselfly fauna; the River Kird has not been systematically surveyed for these insects. In the summer of 2017 we were able to survey over 5km of the lower reaches of the River Kird, from Kirdford to the point where it reaches the River Arun. -

Impact of Environmental Changes on the Behavioral Diversity of the Odonata (Insecta) in the Amazon Bethânia O
www.nature.com/scientificreports OPEN Impact of environmental changes on the behavioral diversity of the Odonata (Insecta) in the Amazon Bethânia O. de Resende1,2*, Victor Rennan S. Ferreira1,2, Leandro S. Brasil1, Lenize B. Calvão2,7, Thiago P. Mendes1,6, Fernando G. de Carvalho1,2, Cristian C. Mendoza‑Penagos1, Rafael C. Bastos1,2, Joás S. Brito1,2, José Max B. Oliveira‑Junior2,3, Karina Dias‑Silva2, Ana Luiza‑Andrade1, Rhainer Guillermo4, Adolfo Cordero‑Rivera5 & Leandro Juen1,2 The odonates are insects that have a wide range of reproductive, ritualized territorial, and aggressive behaviors. Changes in behavior are the frst response of most odonate species to environmental alterations. In this context, the primary objective of the present study was to assess the efects of environmental alterations resulting from shifts in land use on diferent aspects of the behavioral diversity of adult odonates. Fieldwork was conducted at 92 low‑order streams in two diferent regions of the Brazilian Amazon. To address our main objective, we measured 29 abiotic variables at each stream, together with fve morphological and fve behavioral traits of the resident odonates. The results indicate a loss of behaviors at sites impacted by anthropogenic changes, as well as variation in some morphological/behavioral traits under specifc environmental conditions. We highlight the importance of considering behavioral traits in the development of conservation strategies, given that species with a unique behavioral repertoire may sufer specifc types of extinction pressure. Te enormous variety of behavior exhibited by most animals has inspired human thought, arts, and Science for centuries, from rupestrian paintings to the Greek philosophers. -

From the Ebony Jewelwing Damsel
Comp. Parasitol. 71(2), 2004, pp. 141–153 Calyxocephalus karyopera g. nov., sp. nov. (Eugregarinorida: Actinocephalidae: Actinocephalinae) from the Ebony Jewelwing Damselfly Calopteryx maculata (Zygoptera: Calopterygidae) in Southeast Nebraska, U.S.A.: Implications for Mechanical Prey–Vector Stabilization of Exogenous Gregarine Development RICHARD E. CLOPTON Department of Natural Science, Peru State College, Peru, Nebraska 68421, U.S.A. (e-mail: [email protected]) ABSTRACT: Calyxocephalus karyopera g. nov., sp. nov. (Apicomplexa: Eugregarinorida: Actinocephalidae: Actino- cephalinae) is described from the Ebony Jewelwing Damselfly Calopteryx maculata (Odonata: Zygoptera: Calopteryigidae) collected along Turkey Creek in Johnson County, Nebraska, U.S.A. Calyxocephalus gen. n. is distinguished by the form of the epimerite complex: a terminal thick disk or linearly crateriform sucker with a distal apopetalus calyx of petaloid lobes and a short intercalating diamerite (less than half of the total holdfast length). The epimerite complex is conspicuous until association and syzygy. Association occurs immediately before syzygy and is cephalolateral and biassociative. Gametocysts are spherical with a conspicuous hyaline coat. Lacking conspicuous sporoducts they dehisce by simple rupture. Oocysts are axially symmetric, hexagonal dipyramidic in shape with slight polar truncations, bearing 6 equatorial spines, 1 at each equatorial vertex and 6 terminal spines obliquely inserted at each pole, 1 at each vertex created by polar truncation. The ecology of the C. karyopera–C. maculata host–parasite system provides a mechanism for mechanical prey–vector stabilization of exogenous gregarine development and isolation. KEY WORDS: Odonata, Zygoptera, Calopteryx maculata, damselfly, Apicomplexa, Eugregarinida, Actinocephalidae, Actinocephalinae, Calyxocephalus karyopera, new genus, new species, parasite evolution, biodiversity, species isolation, vector, transmission. -
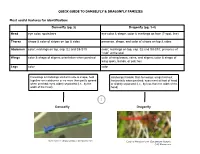
Dragonfly (Pg. 3-4) Head Eye Color
QUICK GUIDE TO DAMSELFLY & DRAGONFLY FAMILIES Most useful features for identification: Damselfly (pg. 2) Dragonfly (pg. 3-4) Head eye color; spots/bars eye color & shape; color & markings on face (T-spot, line) Thorax shape & color of stripes on top & sides presence, shape, and color of stripes on top & sides Abdomen color; markings on top, esp. S2 and S8-S10 color; markings on top, esp. S2 and S8-S10; presence of “club” at the end Wings color & shape of stigma; orientation when perched color of wing bases, veins, and stigma; color & shape of wing spots, bands, or patches Legs color color Forewings & hindwings similar in size & shape, held Hindwings broader than forewings; wings held out together over abdomen or no more than partly spread horizontally when perched; eyes meet at front of head when perched; eyes widely separated (i.e., by the or slightly separated (i.e., by less than the width of the width of the head) head) 1 Damselfly Dragonfly Vivid Dancer (Argia vivida); CAS Mazzacano Cardinal Meadowhawk (Sympetrum illotum); CAS Mazzacano DAMSELFLIES Wings narrow, stalked at base 2 Wings broad, colored, not stalked at base 3 Wings held askew Wings held together Broad-winged Damselfly when perched when perched (Calopterygidae); streams Spreadwing Pond Damsels (Coenagrionidae); (Lestidae); ponds ponds, streams Dancer (Argia); Wings held above abdomen; vivid colors streams 4 Wings held along Bluet (Enallagma); River Jewelwing (Calopteryx aequabilis); abdomen; mostly blue ponds CAS Mazzacano Dark abdomen with blue Forktail (Ischnura); tip; -

The Dragonflies of Lancashire and North Merseyside
Lancashire & Cheshire Fauna Society Registered Charity 500685 www.lacfs.org.uk Publication No. 118 2015 The Dragonflies of Lancashire and North Merseyside Steve White and Philip H. Smith 2 Lancashire & Cheshire Fauna Society The Dragonflies of Lancashire and North Merseyside Steve White and Philip H. Smith Front cover: Banded Demoiselle, Downholland Brook, Formby (Trevor Davenport) Back cover: Common Darter, Seaforth Nature Reserve (Steve Young) Published in 2015 by the Lancashire and Cheshire Fauna Society, Rishton, Lancashire Recommended citation: White, S.J. & Smith, P.H. 2015. The Dragonflies of Lancashire and North Merseyside. Lancashire & Cheshire Fauna Society. Rishton. Lancashire & Cheshire Fauna Society Printed by CPL Design + Print. CONTENTS Acknowledgements 4 Introduction 5 Factors affecting Dragonfly Distribution 9 Main Habitats and Sites 18 SPECIES ACCOUNTS 1 Damselflies Emerald Damselfly Lestes sponsa Banded Demoiselle Calopteryx splendens 5 Beautiful Demoiselle Calopteryx virgo 9 Azure DamselflyCoenagrion puella 40 Common Blue DamselflyEnallagma cyathigerum 44 Red-eyed Damselfly Erythromma najas 47 Blue-tailed Damselfly Ischnura elegans 49 Large Red DamselflyPyrrhosoma nymphula 5 Dragonflies Southern Hawker Aeshna cyanea 56 Brown Hawker Aeshna grandis 59 Common Hawker Aeshna juncea 62 Migrant Hawker Aeshna mixta 65 Emperor DragonflyAnax imperator 69 Lesser Emperor Anax parthenope 7 Hairy Dragonfly Brachytron pratense 7 Golden-ringed DragonflyCordulegaster boltonii 74 Broad-bodied Chaser Libellula depressa 76 Four-spotted -

Calopteryx Splendens, C. Virgo) During Social Interaction: a Signal Or a Symptom?
Russian Entomol. J. 25(1): 103–120 © RUSSIAN ENTOMOLOGICAL JOURNAL, 2016 Behaviour of two demoiselle species (Calopteryx splendens, C. virgo) during social interaction: a signal or a symptom? Ïîâåäåíèå äâóõ âèäîâ ðàâíîêðûëûõ ñòðåêîç êðàñîòêè áëåñòÿùåé Calopteryx splendens Harris, 1780 è êðàñîòêè-äåâóøêè C. virgo Linnaeus, 1758 (Odonata, Calopterygidae) E.N. Panov1*, A.S. Opaev1, E.Yu. Pavlova1, V.A. Nepomnyashchikh2 Å.Í. Ïàíîâ1*, À.Ñ. Îïàåâ1, Å.Þ. Ïàâëîâà1, Â.À. Íåïîìíÿùèõ2 1 A.N. Severtsov Institute of Ecology and Evolution, Russian Academy of Sciences, Leninsky prosp., 33, Moscow, 119071 Russia. 1 Институт проблем экологии и эволюции им. А.Н. Северцова РАН, Ленинский просп., 33, Москва 119071 Россия. 2 Institute of Biology of Inland Waters, Russian Academy of Sciences, Borok, 152742 Russia. 2 Институт биологии внутренних вод РАН, п. Борок, Ярославская область, 152742 Россия. * Corresponding author. E-mail: [email protected] KEY WORDS: damselfly, agonistic behaviour, evolution of courtship behaviour, sexual selection. КЛЮЧЕВЫЕ СЛОВА: стрекоза, агонистическое поведение, эволюция поведения ухаживания, половой отбор. ABSTRACT. Based on the analysis of video record- ного характера. Подвержены серьезной критике ings, obtained during five field seasons, we suggest a трактовки функциональных объяснений поведения pattern of analytical description of damselfly (Zygoptera: стрекоз, господствующие в современной литерату- Calopterygidae) behaviour in a broad spectrum of so- ре по этим насекомым и основанные на откровен- cial contexts. The arrays of motor coordinations and ном антропоморфизме. Этому подходу противопо- their place in social interactions are studied compara- ставлен совершенно иной, призывающий к возвра- tively and in the temporal dynamic. The first description ту к оставленным в последние десятилетия методам of the full set of behaviours of Banded Demoiselles и принципам, разработанным ранее в рамках поле- Calopteryx splendens is given; the respective data for вой и аналитической этологии. -

A Model for the Web-Based Delivery of Natural History Information and Citizen Science
OdonataCentral.com: A Model for the Web-based Delivery of Natural History Information and Citizen Science J. C. Abbott, D. Broglie he World Wide Web can be as cluttered web technology to digitally deliver natural history and confusing as the house of that ec- information. Tcentric aunt you never wanted to visit. Knickknacks from Tampa were strewn across the History kitchen floor, assorted Hummels were stocked in November 2004 saw the quiet launch of Odo- the medicine cabinet, and piles of old National nataCentral (http://www.odonatacentral.com) (Fig. Geographic magazines teetered precariously to 1), a web site sponsored by the Texas Memorial the ceiling. Navigating the house was difficult and Museum at the University of Texas at Austin that perilous. Nothing was ever in its correct place, relies on the novel incorporation of existing World but then again, there never was a correct place for Wide Web, database, and geographic information anything either. system (GIS) technologies to produce a dynamic, For biologists, trying to find accurate, up-to- interactive field guide and web site for the dragon- date natural history and distribution information flies and damselflies of North America. is an equally frustrating venture. When one does The traditional approach to online field guides find a legitimate field guide, it will probably con- relies heavily on static (not database-generated) sist of outdated, kludgy, or static species lists and checklists, species descriptions, and distribution distribution maps. The prevalence of such sites maps. Thus, whenever an author needs to update seems counterintuitive to our modern mantra that one county record, many or all of the regional and computer advances are supposed to automate and global pages must be edited manually.