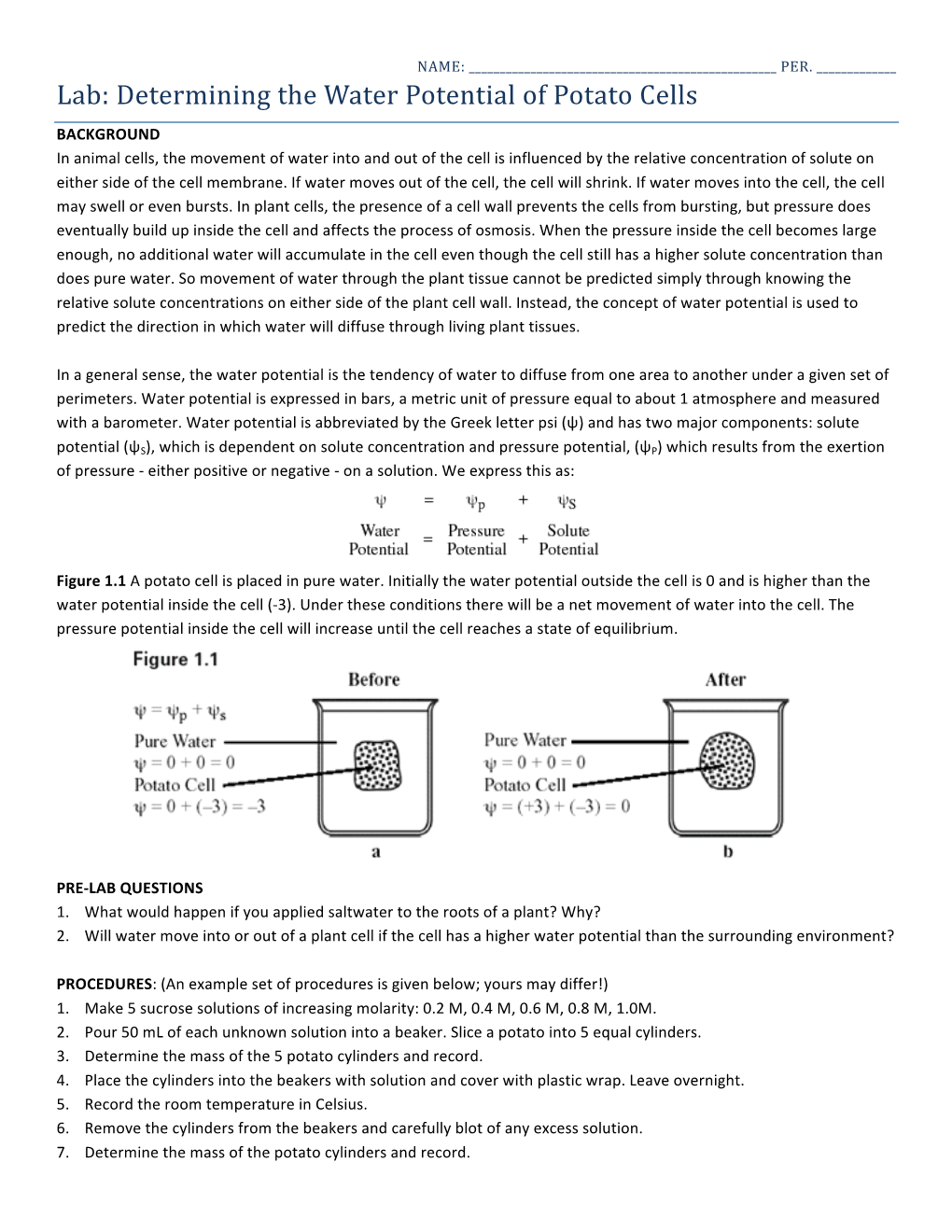
Lab: Determining the Water Potential of Potato Cells
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Water Content Concepts and Measurement Methods Suat Irmak, Professor, Soil and Water Resources and Irrigation Engineering
EC3046 December 2019 Soil- Water Potential and Soil- Water Content Concepts and Measurement Methods Suat Irmak, Professor, Soil and Water Resources and Irrigation Engineering Soil- water status is a critical and rapidly changing tion management. In addition, it is important for studying variable that determines and impacts numerous important soil- water movement, chemical transport, crop water stress, factors in production fields such as crop emergence and evapotranspiration, hydrologic and crop modeling, soil phys- growth, water management, water and crop yield productiv- ics, water resources management, climate change impacts ity relationships, and within- field hydrologic balances. Thus, on agricultural water management and crop productivity, its accurate determination dictates and impacts the success of meteorological studies, yield forecasting, water run- off and water management and related agricultural operations. This, run- on, infiltration studies, field traffic and within- field work in turn, affects the attainment of potential yield, as well as the ability and soil- compaction studies, aridity indices, and other reduction of water losses and chemical leaching. Maintaining agricultural and ecosystem functions and practices. Effective optimum soil moisture in the crop root zone also strongly in- irrigation management requires the knowledge of “when” fluences optimum nitrogen (N) uptake by plants, which helps and “how much” water to apply to optimize crop production. to reduce N leaching. Numerous soil moisture measurement Some of the most effective irrigation management decisions technologies are available. None of the methods, however, also include “how” to apply the irrigation water for most are perfectly suited to all operational conditions as each has effective productivity under different climate, soil, crop, and drawbacks and advantages, depending on the application management practices to reduce unbeneficial water losses conditions. -

Summary a Plant Is an Integrated System Which: 1
Summary A plant is an integrated system which: 1. Obtains water and nutrients from the soil. 2. Transports them 3. Combines the H2O with CO2 to make sugar. 4. Exports sugar to where it’s needed Today, we’ll start to go over how this occurs Transport in Plants – Outline I.I. PlantPlant waterwater needsneeds II.II. TransportTransport ofof waterwater andand mineralsminerals A.A. FromFrom SoilSoil intointo RootsRoots B.B. FromFrom RootsRoots toto leavesleaves C.C. StomataStomata andand transpirationtranspiration WhyWhy dodo plantsplants needneed soso muchmuch water?water? TheThe importanceimportance ofof waterwater potential,potential, pressure,pressure, solutessolutes andand osmosisosmosis inin movingmoving water…water… Transport in Plants 1.1. AnimalsAnimals havehave circulatorycirculatory systems.systems. 2.2. VascularVascular plantsplants havehave oneone wayway systems.systems. Transport in Plants •• OneOne wayway systems:systems: plantsplants needneed aa lotlot moremore waterwater thanthan samesame sizedsized animals.animals. •• AA sunflowersunflower plantplant “drinks”“drinks” andand “perspires”“perspires” 1717 timestimes asas muchmuch asas aa human,human, perper unitunit ofof mass.mass. Transport of water and minerals in Plants WaterWater isis goodgood forfor plants:plants: 1.1. UsedUsed withwith CO2CO2 inin photosynthesisphotosynthesis toto makemake “food”.“food”. 2.2. TheThe “blood”“blood” ofof plantsplants –– circulationcirculation (used(used toto movemove stuffstuff around).around). 3.3. EvaporativeEvaporative coolingcooling. -

Transport of Water and Solutes in Plants
Transport of Water and Solutes in Plants Water and Solute Potential Water potential is the measure of potential energy in water and drives the movement of water through plants. LEARNING OBJECTIVES Describe the water and solute potential in plants Key Points Plants use water potential to transport water to the leaves so that photosynthesis can take place. Water potential is a measure of the potential energy in water as well as the difference between the potential in a given water sample and pure water. Water potential is represented by the equation Ψsystem = Ψtotal = Ψs + Ψp + Ψg + Ψm. Water always moves from the system with a higher water potential to the system with a lower water potential. Solute potential (Ψs) decreases with increasing solute concentration; a decrease in Ψs causes a decrease in the total water potential. The internal water potential of a plant cell is more negative than pure water; this causes water to move from the soil into plant roots via osmosis.. Key Terms solute potential: (osmotic potential) pressure which needs to be applied to a solution to prevent the inward flow of water across a semipermeable membrane transpiration: the loss of water by evaporation in terrestrial plants, especially through the stomata; accompanied by a corresponding uptake from the roots water potential: the potential energy of water per unit volume; designated by ψ Water Potential Plants are phenomenal hydraulic engineers. Using only the basic laws of physics and the simple manipulation of potential energy, plants can move water to the top of a 116-meter-tall tree. Plants can also use hydraulics to generate enough force to split rocks and buckle sidewalks. -

Evaluation of Midday Stem Water Potential Amd Soil Moisture Measurement for Irrigation Scheduling in Walnut
RELATIONSHIPS BETWEEN MIDDAY STEM WATER POTENTIAL, SOIL MOISTURE MEASUREMENT, AND WALNUT SHOOT GROWTH Allan Fulton, Richard Buchner, Cayle Little, Cyndi Gilles, Joe Grant, Bruce Lampinen, Ken Shackel, Larry Schwankl, Terry Prichard, and Dan Rivers. ABSTRACT Measuring midday stem water potential (SWP) with a pressure chamber is an emerging technology for guiding irrigation management in walnut. This study looks at relationships between SWP, soil moisture measurement, and walnut shoot growth in one walnut orchard in Tehama County. The goal was to evaluate the merits for adopting this technology at the farm level. SWP did not correlate well to soil water content measurement without converting the soil water content to soil water depletion. SWP and soil water depletion correlated reasonably well in this study indicating SWP measurement is a valid indicator of orchard water status. Both SWP and soil water depletion correlated equally with average monthly shoot growth. While adaptability of SWP for on-farm use has been questioned for pragmatic reasons such as limiting measurement to mid afternoon hours and in the heat of the day, it may be more precise and practical than measurement of soil water depletion because it is a direct measurement of orchard water status and is not contingent upon the validity of assumptions made to estimate soil water depletion. INTRODUCTION Midday stem water potential (SWP) measurement with a pressure chamber is a relatively new plant-based tool available to assist walnut growers with making irrigation management decisions. In general, growers are more accustom to adopting water budget methods where climate based estimates of crop evapotranspiration (ETc) are used or methods of soil moisture monitoring to guide their irrigation decisions. -

Soil Water Status: Content and Potential by Jim Bilskie, Ph.D
Soil water status: content and potential By Jim Bilskie, Ph.D. The fundamental forces acting on soil Campbell Scientific, Inc. mwet 94 g water are gravitational, matric, and osmot- ic. Water molecules have energy by mdry 78 g he state of water in soil is described virtue of position in the gravitational force T in terms of the amount of water and sample volume 60 cm3 field just as all matter has potential ener- the energy associated with the forces gy. This energy component is described which hold the water in the soil. The by the gravitational potential component amount of water is defined by water con- of the total water potential. The influence mwater 94gg− 78 tent and the energy state of the water is θ == = −1 of gravitational potential is easily seen g 0. 205 gg the water potential. Plant growth, soil msoil 78g when attractive forces between water and temperature, chemical transport, and soil are less than the gravitational forces ground water recharge are all dependent acting on the water molecule and water m on the state of water in the soil. While dry 78 g − moves downward. ρ ===13. gcm 3 there is a unique relationship between bulk volume 60 cm3 The matrix arrangement of soil solid water content and water potential for a particles results in capillary and electro- particular soil, these physical properties static forces and determines the soil water describe the state of the water in soil in θρ∗ matric potential. The magnitude of the g soil − distinctly different manners. It is impor- θ = = 0. -

Osmoregulation in Cotton in Response to Water Stress' I
Plant Physiol. (1981) 67, 484 488 0032-0889/81/67/0484/05/$00.50/0 Osmoregulation in Cotton in Response to Water Stress' I. ALTERATIONS IN PHOTOSYNTHESIS, LEAF CONDUCTANCE, TRANSLOCATION, AND ULTRASTRUCTURE Received for publication June 13, 1980 and in revised form October 13, 1980 ROBERT C. ACKERSON AND RICHARD R. HEBERT Central Research and Development Department, Experimental Station, E. L du Pont de Nemours and Company, Wilmington, Delaware 19898 ABSTRACT paper in this series defines the role of specific cellular carbohy- drates in stress adaptation (2). Cotton plants subjected to a series of water deficits exhibited stress adaptation In the form of osmoregulation when plants were subjected to a subsequent drying cycle. After adaptation, the leaf water potential coincid- MATERIALS AND METHODS ing with zero turgor was considerably lower than in plants that had never Plant Material. Cotton (Gossypium hirsutum L. Tamcot SP37) experienced a water stress. The relationship between leaf turgor and leaf plants were grown from seed in controlled environment facilities water potential depnded O leaf age. as described (3). When the fifth leaf above the cotyledons was Nonstomatal factors severely limited photosynthesis in adapted plants about 75% expanded, one set of plants was subjected to repetitive at high leaf water potential. Nonetheless, adapted plants maintained pho- water stress cycles while another set was well watered. Each stress tosyPnthesis to a much lower leaf water potential than did control plants, in cycle consisted of allowing plants to dehydrate until midday leaf part because of increased stomatal conductance at low leaf water poten- water potentials reached approximately -20 bars. -

Irrigation Scheduling by Soil Water Potential
Irrigation scheduling by soil water potential Clinton C. Shock, Professor and Superintendent, Oregon State University, Malheur Experiment Station, 595 Onion Ave., Ontario, OR, email [email protected] Abstract. One of the fundamental ways to schedule irrigation is through the monitoring and management of soil water potential (SWP). Soil water tension (SWT) is the force necessary for roots to extract water from the soil and growers find it easier to deal with the positive units of SWT rather than the negative units of SWP. With the invention of tensiometers, SWT measurements have been used to schedule irrigation. There are seven different types of field instruments used to measure SWT, either directly or indirectly. Specific SWT criteria for irrigation scheduling have been developed for the production of individual vegetable crops, field crops, trees, shrubs, and nursery crops and for the management of landscape plants. A review of the known SWT criteria for irrigation scheduling will be presented. Keywords. Soil water tension, Tensiometer, Granular matrix sensor Why Measure Soil Water Tension The lack of water in plant-top tissues is transmitted through the plant, down into the roots, and into the soil. The measurement of soil water tension can be closely related to the stress experienced by the plant tissues, and in these conditions irrigation scheduling based on soil water tension can be directly related to plant performance. The use of a soil water tension measuring device provides a continuous measurement analogous to the force (suction) necessary to extract water from the soil. When growers irrigate too infrequently and with too much water, product yield and quality is lost (Tjosvold and Schulbach 1991) and water and nutrients are lost, with the potential of environmental harm. -

Moisture Content-Water Potential Characteristic
MOISTURE CONTENT-WATER POTENTIAL CHARACTERISTIC CURVES FOR RED OAK AND LOBLOLLY PINE Jian Zhangf Former Graduate Research Assistant Department of Forest Resources Clemson University Clemson, SC 29634-1003 and Perry N. Peraltat Assistant Professor Department of Wood and Paper Science North Carolina State University Raleigh, NC 27695-8005 (Received September 1998) ABSTRACT This report describes the results of a study performed to measure the water potential of loblolly pine and red oak over the full range of moisture content during desorption. The matric potential as measured by the tension plate, pressure plate, and pressure membrane methods exhibited good con- tinuity with the total water potential as measured by the isopiestic method. This not only proves the validity of the water potential measurements but also shows that the osmotic potential component of the total water potential is negligible at low moisture content. The characteristic curves allow char- acterization of watcr in wood at high moisturc contents and thus avoid the need to extrapolate sorption isothcrm beyond the 98% relativc humidity level as was done in prcvious sorption studics. The results also show that, at a given watcr potential, the moisturc contents of both species decrease with a rise in temperature. This may be due partly to the temperature dcpendence of the surface tension of water and to the fact that entrapped air expands when heated, thus displacing water out of the capillaries. The temperature dependence of water potential was used to calculate the enthalpy change, the free energy change, and the product of absolute temperature and entropy change associated with moisture sorption. -

Osmoregulation and Other Survival Strategies Deployed by The
Pakistan Journal of Marine Sciences, Vol.1(1), 73-86, 1992 REVIEW ARTICLE COPING WITH EXCESS SALT IN THEIR GROWTH ENVI RONMENTS: OSMOREGULATION AND OTHER SURVIVAL STRA1EGIES DEPLOYED BY THE MANGROVES. Saiyed I. Ahmed School of Oceanography, University of Washington, Seattle, WA 98195, U.S.A. INTRODUCI'ION Mangroves are defined here as a collection of woody plants and the associated fauna and flora that use a coastal depositional environment. Mangrove forests consist of plant communities that belong to many different genera and families that are not always related phylogenetically. However, they share some common characteristics based upon physiological, reproductive and morphological adaptations that enable them to grow in a broad range of coastal environments in the tropical and subtropical areas of the world. By this definition, they occupy the interface between the land and the sea (Walsh, 1974). Throughout the world, approximately 54 species of plants belonging to about 20 genera in 16 families have been recognized as belonging to the mangroves (Tomlinson, 1986). The mangrove forests of Pakistan consist of 8 species in the Indus River delta region and 5 species along the Makran Coast. However, the species of the genusAvicennia are the dominant members in both these areas and represent 90-95% of the total mangrove vegetation (Snedaker, 1984). Jennings and Bird (1967) have described the six most important geomorphological characteristics that affect estuaries, and they are: (1) aridity, (2) wave energy, (3) tidal conditions, ( 4) sedimentation, (5) mineralogy and (6) neotectonic effects. All of these directly or indirectly affect the establishment of mangroves. Walsh (1974) and Chapman (1975, 1977) have described in all seven characteristics that may be consid ered as essential requisites for mangroves on a world-wide basis; (1) air temperature (within a restricted range), (2) mud substrate, (3) protection, (4) salt water, (5) tidal range, (6) ocean currents, and (7) shallow shores. -

Effects of Leaflet Orientation on Transpiration Rates and Water Potentials of Oxalis Montana
Effects of Leaflet Orientation on Transpiration Rates and Water Potentials of Oxalis montana Hope K. Comerro and Dr. George Briggs Abstract- The leaflets of Oxalis montana (wood sorrel) show reversible leaflet movements with leaflets moving from a horizontal position to a vertical one in response to direct solar radiation. We studied the possibility that this response was (1) hydropassive, resulting from decreased water potential caused by higher transpiration rates in direct sunlight and (2) a mechanism to reduce water loss under conditions of high radiation. Using a lysimeter technique, the transpiration rate of O. montana with vertical leaflets was found to be higher than the transpiration rate of plants with leaflets in a horizontal position. The water potential of leaves with leaflets in a vertical orientation was significantly lower than the water potential of leaves with horizontal leaflets. These results do not support the hypothesis that leaflet movement is a mechanism to reduce water loss, but they are consistent with a hydropassive response, one where leaflet movement results from lower leaf water potential brought about by increased transpiration. Introduction Oxalis montana is a herbaceous perennial trifoliate leaves of Oxalis fold downward, common in the temperate forests of central in response to direct sunlight, from a New England to Wisconsin, and north into horizontal position in the shade, to a Canada. It has a scaly, woody underground vertical position (Figure 1.) Similar Figure 1 Leaflets Horizontal Leaflets Vertical rhizome that sends up clover shaped leaves responses have been reported for Oxalis in the spring, which persist for the summer regnellii, a species from Europe (Pedersen then die back in the fall. -

Soil Water Potential Control of the Relationship Between Moisture and Greenhouse Gas Fluxes in Corn-Soybean Field
Climate 2015, 3, 689-696; doi:10.3390/cli3030689 OPEN ACCESS climate ISSN 2225-1154 www.mdpi.com/journal/climate Article Soil Water Potential Control of the Relationship between Moisture and Greenhouse Gas Fluxes in Corn-Soybean Field Dinesh Panday 1 and Nsalambi V. Nkongolo 1,2,* 1 Department of Agriculture and Environmental Sciences, Lincoln University, Jefferson City, MO 65102-0029, USA; E-Mail: [email protected] 2 Institut Facultaire des Sciences Agronomiques (IFA) de Yangambi, BP 28 Yangambi, Republique Democratique du Congo * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-573-681-5397; Fax: +1-573-681-5154. Academic Editors: Nir Y. Krakauer, Tarendra Lakhankar, Soni M. Pradhanang, Vishnu Pandey and Madan Lall Shrestha Received: 11 March 2015 / Accepted: 11 August 2015 / Published: 19 August 2015 Abstract: Soil water potential (Ψ) controls the dynamics of water in soils and can therefore affect greenhouse gas fluxes. We examined the relationship between soil moisture content (θ) at five different levels of water potential (Ψ = 0, −0.05, −0.1, −0.33 and −15 bar) and greenhouse gas (carbon dioxide, CO2; nitrous oxide, N2O and methane, CH4) fluxes. The study was conducted in 2011 in a silt loam soil at Freeman farm of Lincoln University. Soil samples were collected at two depths: 0–10 and 10–20 cm and their bulk densities were measured. Samples were later saturated then brought into a pressure plate for measurements of Ψ and θ. Soil air samples for greenhouse gas flux analyses were collected using static and vented chambers, 30 cm in height and 20 cm in diameter. -

Demonstrating Osmotic Potential: One Factor in the Plant Water Potential Equation
Tested Studies for Laboratory Teaching Proceedings of the Association for Biology Laboratory Education Vol. 36, Article 29, 2015 Demonstrating Osmotic Potential: One Factor in the Plant Water Potential Equation Rosemary H. Ford Washington College, Biology Department, 300 Washington Ave., Chestertown MD 21620 USA ([email protected]) Water potential in plants depends on osmotic and pressure potentials. Pressure potential is easily understand by monitoring changes in tissue mass when immersed in various sugar solutions. Osmotic potential can be visualized by comparing the cell sap’s density with these same sugar solutions in which it will rise, sink, or hover, in which the latter represents the concentration of equal density suggesting an isosmotic condition. Students can use this method to compare the density of various plant tissues (e.g. potato vs apple) or construct a Höfler plot using the Van’t Hoft equation to illustrate relationships between osmotic and pressure potential. Firstpage Keywords: water potential, osmotic potential, plant physiology Introduction In General Biology, a typical laboratory about osmosis might use plant tissue or alga placed in various sugar con- centrations to observe the response. Changes in the cells are using an osmometer, the instruments are expensive and do easily visualized since the cells walls allow either pressure to not allow students to visualize the effect of solute concentra- build within the cell (increased turgor pressure) when placed tions, which can be demonstrated by comparing density of in hypotonic solutions and the cytoplasm to shrink (plasmol- cell sap with known concentrations of sugar. It is important ysis) with the loss of water in hypertonic solutions.