Ia-- ~~@ CH: (1) (A) Colorado State University
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Common Names for Selected Aromatic Groups
More Nomenclature: Common Names for Selected Aromatic Groups Phenyl group = or Ph = C6H5 = Aryl = Ar = aromatic group. It is a broad term, and includes any aromatic rings. Benzyl = Bn = It has a -CH2- (methylene) group attached to the benzene ring. This group can be used to name particular compounds, such as the one shown below. This compound has chlorine attached to a benzyl group, therefore it is called benzyl chloride. Benzoyl = Bz = . This is different from benzyl group (there is an extra “o” in the name). It has a carbonyl attached to the benzene ring instead of a methylene group. For example, is named benzoyl chloride. Therefore, it is sometimes helpful to recognize a common structure in order to name a compound. Example: Nomenclature: 3-phenylpentane Example: This is Amaize. It is used to enhance the yield of corn production. The systematic name for this compound is 2,4-dinitro-6-(1-methylpropyl)phenol. Polynuclear Aromatic Compounds Aromatic rings can fuse together to form polynuclear aromatic compounds. Example: It is two benzene rings fused together, and it is aromatic. The electrons are delocalized in both rings (think about all of its resonance form). Example: This compound is also aromatic, including the ring in the middle. All carbons are sp2 hybridized and the electron density is shared across all 5 rings. Example: DDT is an insecticide and helped to wipe out malaria in many parts of the world. Consequently, the person who discovered it (Muller) won the Nobel Prize in 1942. The systematic name for this compound is 1,1,1-trichloro-2,2-bis-(4-chlorophenyl)ethane. -

Chapter 21 the Chemistry of Carboxylic Acid Derivatives
Instructor Supplemental Solutions to Problems © 2010 Roberts and Company Publishers Chapter 21 The Chemistry of Carboxylic Acid Derivatives Solutions to In-Text Problems 21.1 (b) (d) (e) (h) 21.2 (a) butanenitrile (common: butyronitrile) (c) isopentyl 3-methylbutanoate (common: isoamyl isovalerate) The isoamyl group is the same as an isopentyl or 3-methylbutyl group: (d) N,N-dimethylbenzamide 21.3 The E and Z conformations of N-acetylproline: 21.5 As shown by the data above the problem, a carboxylic acid has a higher boiling point than an ester because it can both donate and accept hydrogen bonds within its liquid state; hydrogen bonding does not occur in the ester. Consequently, pentanoic acid (valeric acid) has a higher boiling point than methyl butanoate. Here are the actual data: INSTRUCTOR SUPPLEMENTAL SOLUTIONS TO PROBLEMS • CHAPTER 21 2 21.7 (a) The carbonyl absorption of the ester occurs at higher frequency, and only the carboxylic acid has the characteristic strong, broad O—H stretching absorption in 2400–3600 cm–1 region. (d) In N-methylpropanamide, the N-methyl group is a doublet at about d 3. N-Ethylacetamide has no doublet resonances. In N-methylpropanamide, the a-protons are a quartet near d 2.5. In N-ethylacetamide, the a- protons are a singlet at d 2. The NMR spectrum of N-methylpropanamide has no singlets. 21.9 (a) The first ester is more basic because its conjugate acid is stabilized not only by resonance interaction with the ester oxygen, but also by resonance interaction with the double bond; that is, the conjugate acid of the first ester has one more important resonance structure than the conjugate acid of the second. -

Metabolic-Hydroxy and Carboxy Functionalization of Alkyl Moieties in Drug Molecules: Prediction of Structure Influence and Pharmacologic Activity
molecules Review Metabolic-Hydroxy and Carboxy Functionalization of Alkyl Moieties in Drug Molecules: Prediction of Structure Influence and Pharmacologic Activity Babiker M. El-Haj 1,* and Samrein B.M. Ahmed 2 1 Department of Pharmaceutical Sciences, College of Pharmacy and Health Sciences, University of Science and Technology of Fujairah, Fufairah 00971, UAE 2 College of Medicine, Sharjah Institute for Medical Research, University of Sharjah, Sharjah 00971, UAE; [email protected] * Correspondence: [email protected] Received: 6 February 2020; Accepted: 7 April 2020; Published: 22 April 2020 Abstract: Alkyl moieties—open chain or cyclic, linear, or branched—are common in drug molecules. The hydrophobicity of alkyl moieties in drug molecules is modified by metabolic hydroxy functionalization via free-radical intermediates to give primary, secondary, or tertiary alcohols depending on the class of the substrate carbon. The hydroxymethyl groups resulting from the functionalization of methyl groups are mostly oxidized further to carboxyl groups to give carboxy metabolites. As observed from the surveyed cases in this review, hydroxy functionalization leads to loss, attenuation, or retention of pharmacologic activity with respect to the parent drug. On the other hand, carboxy functionalization leads to a loss of activity with the exception of only a few cases in which activity is retained. The exceptions are those groups in which the carboxy functionalization occurs at a position distant from a well-defined primary pharmacophore. Some hydroxy metabolites, which are equiactive with their parent drugs, have been developed into ester prodrugs while carboxy metabolites, which are equiactive to their parent drugs, have been developed into drugs as per se. -

Methyl Substitution Effects on the Proton Chemical Shifts in Benzene *
Methyl Substitution Effects on the Proton Chemical Shifts in Benzene * G. S. REDDY E. I. du Pont de Nemours & Company, Inc. Explosives Department, Eastern Laboratory, Gibbstown, New Jersey, U.S.A. (Z. Naturforschg. 21 a, 609—615 [1966] ; received 16 December 1965) Methyl substitution effects on aromatic and methyl proton chemical shifts in several mono-, di-, and trimethyl benzenes are studied. A new method for obtaining the changes in the ring proton chemical shifts from those of methyl proton shifts at the corresponding positions is used. The extra jr-electron densities in toluene are calculated using the already known relation between the jr-elec- tron densities and the proton shifts in aromatic systems. An inverse relationship is obtained between the ionization potentials and the total methyl effects on the chemical shifts in this series of com- pounds as one would expect. Dipole moment of toluene is calculated, and a reasonably good agree- ment is found between the experimentally observed and the calculated dipole moment. Several efforts have been made from time to Considerable work has also been done in estimat- time to study the substitution effects on chemical ing ir-electron densities from chemical shift meas- shifts and coupling constants. One of the earliest urements in unsaturated systems. This study in- attempts in this line are those of CAVANAUGH and volves extension of the substitution effects and also DAILEY 1 who tried to study the effect of multiple estimating jr-electron densities in methyl benzenes. methyl substitution in methane. They encountered Eight mono-, di-, and trimethyl substituted benzenes negative shifts contrary to expectations based on have been studied, and a new technique has been inductive and hyperconjugative effects of the methyl deployed to obtain the methyl substitution effects group which eventually was attributed to the an- on the chemical shifts of ring protons from proton isotropy effect of the added C — C bonds 2-7. -

Physicochemical Properties of Organic Medicinal Agents
Principles of Drug Action 1, Spring 2005, Esters ESTERS AND RELATED CARBOXYLIC ACID DERIVATIVES Jack DeRuiter I. Structure and Preparation Esters are derivatives of carboxylic acids that arise via replacement of the hydroxyl (OH) portion of the acid COOH function with an "ether" moiety (-OR): O O H C C O C O Acid Ester Note that replacement of the acid OH group with an "ether" moiety removes the acidic function from the parent structure (acid) resulting in the formation of non-acidic (neutral, but somewhat polar) compounds (esters). Esters can be sub-classified based on their general structure as aliphatic, aromatic or cyclic (called "lactones") as illustrated by the examples below: O O CH2CH3 CH2CH3 O CH3 O O O Aliphatic Ester Aromatic Ester Cyclic Ester (Lactone) A variety of methods have been developed for the preparation of esters. Most of these methods involve reaction of an alcohol with an "activated carboxylic acid" compound (i.e. acid chloride): O O H C C X OC C O X- Ester "Activated" acid (X=Cl) Alcohol (Electrophile) (Nucleophile) The ester functionality does not introduce a center of asymmetry and thus optical and geometric isomerism does not result from the presence of this functional group. The ester functionality (the carbonyl and ether oxygen) is composed of an sp2 hybridized carbon so it cannot be chiral, and since there is free rotation about the ether bond geometric isomerism also is not possible at the sp2 center. 1 Principles of Drug Action 1, Spring 2005, Esters II. Solubility of Esters Esters contain carbonyl (C=O) and ether (O-C) dipoles arising from covalent bonding between electronegative oxygen atoms and electronically neutral carbon atoms. -

Halogenated Ether, Alcohol, and Alkane Anesthetics Activate TASK-3 Tandem Pore Potassium Channels Likely Through a Common Mechanism S
Supplemental material to this article can be found at: http://molpharm.aspetjournals.org/content/suppl/2017/03/21/mol.117.108290.DC1 1521-0111/91/6/620–629$25.00 https://doi.org/10.1124/mol.117.108290 MOLECULAR PHARMACOLOGY Mol Pharmacol 91:620–629, June 2017 Copyright ª 2017 by The American Society for Pharmacology and Experimental Therapeutics Halogenated Ether, Alcohol, and Alkane Anesthetics Activate TASK-3 Tandem Pore Potassium Channels Likely through a Common Mechanism s Anita Luethy, James D. Boghosian, Rithu Srikantha, and Joseph F. Cotten Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts (A.L., J.D.B., and J.F.C.); Department of Anesthesia, Kantonsspital Aarau, Aarau, Switzerland (A.L.); Carver College of Medicine, University of Iowa, Iowa City, Iowa (R.S.) Received January 7, 2017; accepted March 20, 2017 Downloaded from ABSTRACT The TWIK-related acid-sensitive potassium channel 3 (TASK-3; hydrate (165% [161–176]) . 2,2-dichloro- . 2-chloro 2,2,2- KCNK9) tandem pore potassium channel function is activated by trifluoroethanol . ethanol. Similarly, carbon tetrabromide (296% halogenated anesthetics through binding at a putative anesthetic- [245–346]), carbon tetrachloride (180% [163–196]), and 1,1,1,3,3,3- binding cavity. To understand the pharmacologic requirements for hexafluoropropanol (200% [194–206]) activate TASK-3, whereas molpharm.aspetjournals.org TASK-3 activation, we studied the concentration–response of the larger carbon tetraiodide and a-chloralose inhibit. Clinical TASK-3 to several anesthetics (isoflurane, desflurane, sevoflurane, agents activate TASK-3 with the following rank order efficacy: halothane, a-chloralose, 2,2,2-trichloroethanol [TCE], and chloral halothane (207% [202–212]) . -

BENZENE AS a LARVICIDE for SCREW WORMS1 the Larval Stage
BENZENE AS A LARVICIDE FOR SCREW WORMS1 By D. C. PARMAN Assistant Entomologist, Investigations of Insects Affecting the Health of Animals, Bureau of Entomology, United States Department of Agriculture INTRODUCTION The larval stage of CocMiomyia macellaria Fab., generally known amonff stock raisers in the Southwest as the screw worm, causes con- siderable loss to the livestock industry, estimated as high as $5,000,000 in some years. It has been apparent that the larvicides used to kill the worms are either toxic to the animal or at least in most cases detrimental to the healing of the wounds. This toxicity was at first attributed to the screw worm, but as many cases were ob- served where the animal was practically consumed by the larvae and still lived until the loss of olood or injury to some vital organ brought de^th, it was surmised that the treatments with larvicides were the cause of many deaths. During the summer of 1916 syste- matic work was begun to find a more efficient larvicide than the phenols and chloroform which were generally used. At first an attempt was made to add something to these larvicides to counteract the toxic properties. As this was not successful it was deemed best to look for a chemical that might be used with more satis- factory results. Several chemical groups were studied for possible larvicides. EXPERIMENTAL PROCEDURE All available chemicals with possible larvicidal value were selected for laboratory tests to determine whether they would kill the larvae of the screw-worm fly. The first tests were made by pouring the chemical on a number of larvae in a tube, or dusting on just enough to cover the larvae. -

Functional Groups Kimberly Hatch Harrison
Functional Groups Kimberly Hatch Harrison Functional groups are those small chemical species you see hanging off the outside of a molecule. Just a handful of these functional groups de- termine most of the chemical reactions that happen between biological molecules. If you memorize the chemical behavior of these functional groups, you’ll be able to predict what kinds of reactions biological molecules can do. You can’t open a lock with a screwdriver–the shape of a screwdriver is quite different from a key, which means it has a dif- ferent function. "Form determines function" is something you’ll hear over and over in biochemistry, and that’s because it’s true. The over- all 3-dimensional shape of a molecule allows it to fit into another molecule, like how a key fits into a lock. But not all keys are the same. You have to look closely at the teeth of a key to see which lock it can open. Similarly, you need to look at the details of the outside of a molecule to understand what kinds of chemical interactions it can do with other molecules. How the carbon skeleton of a biological molecule is folded up de- termines its general 3D shape. So that’s one level of understanding– this molecule looks like a key, this one looks like a lock, etc. But then you must look closer, at the surface details, to understand exactly which key, exactly what kind of lock. When you examine the outside of a biological molecule, you can identify which functional groups are standing out on its surface, like little flags. -

Chemistry and Physics of Lipids Effects of Ether Vs. Ester Linkage On
Chemistry and Physics of Lipids 160 (2009) 33–44 Contents lists available at ScienceDirect Chemistry and Physics of Lipids journal homepage: www.elsevier.com/locate/chemphyslip Effects of ether vs. ester linkage on lipid bilayer structure and water permeability S. Deren Guler a, D. Dipon Ghosh b, Jianjun Pan a, John C. Mathai c, Mark L. Zeidel c, John F. Nagle a,b, Stephanie Tristram-Nagle a,∗ a Department of Physics, Carnegie Mellon University, Pittsburgh, PA 15213, USA b Department of Biological Sciences, Carnegie Mellon University, Pittsburgh, PA 15213, USA c Department of Medicine, Beth Israel Deaconess Medical Center and Harvard Medical School, Cambridge, MA 02139, USA article info abstract Article history: The structure and water permeability of bilayers composed of the ether-linked lipid, dihexadecylphos- Received 29 January 2009 phatidylcholine (DHPC), were studied and compared with the ester-linked lipid, dipalmitoylphosphadit- Received in revised form 29 March 2009 dylcholine (DPPC). Wide angle X-ray scattering on oriented bilayers in the fluid phase indicate that the Accepted 26 April 2009 area per lipid A is slightly larger for DHPC than for DPPC. Low angle X-ray scattering yields A = 65.1 Å2 for Available online 3 May 2009 ◦ −13 DHPC at 48 C. LAXS data provide the bending modulus, KC = 4.2 × 10 erg, and the Hamaker parame- ter H =7.2× 10−14 erg for the van der Waals attractive interaction between neighboring bilayers. For the Keywords: low temperature phases with ordered hydrocarbon chains, we confirm the transition from a tilted L Ether lipid ◦ gel phase to an untilted, interdigitated LI phase as the sample hydrates at 20 C. -

Organic Chemistry Nomenclature Guide
Chemistry 222 Organic Chemistry Nomenclature Guide Many molecules in organic chemistry can be named using alkyl groups. MEMORIZE THEM! Common Alkyl (R) Groups Number of Carbons Formula Name 1 -CH3 methyl 2 -CH2CH3 ethyl 3 -CH2CH2CH3 propyl 4 -CH2CH2CH2CH3 butyl 5 -CH2CH2CH2CH2CH3 pentyl 6 -CH2(CH2)4CH3 hexyl 7 -CH2(CH2)5CH3 heptyl 8 -CH2(CH2)6CH3 octyl 9 -CH2(CH2)7CH3 nonyl Alkyl groups are generically referred to as R-groups, where R could be a methyl group, ethyl group, octyl group, etc. Organic compounds are often lumped into families or classes of compounds. The classes we will study this term include the following: R H R O H R O R R X Alcohols Ethers Alkanes Cycloalkanes Alkyl Halides or haloalkanes O O C C C C R C R C R H Ketones Aldehydes Alkynes Alkenes Aromatics H O O O All of these families are detailed in the R N R C R C R C pages that follow. H O H O R NH2 Amides Amines Carboxylic Acids Esters Page IV-20-1 / Organic Chemistry Nomenclature Guide Alkanes Elemental Formula: CnH2n+2 H Nomenclature Guidelines: -yl on alkyl group, +ane to ending Notes: An alkane is an alkyl group plus a hydrogen, often referenced as R-H. H C H Alkanes contain only carbon and hydrogen atoms in long chains with no rings. Each 3 H carbon atom is sp hybridized. Alkanes make great fuels but are generally unreactive. methane, CH4 Example: CH4 - methane - is a methyl group plus a hydrogen (CH3-H) Example: C2H6 - ethane - is a ethyl group plus a hydrogen (CH3CH2-H) Cycloalkanes H Elemental Formula: CnH2n H Nomenclature Guidelines: cyclo+ -yl on alkyl group, +ane to ending C H Notes: Cycloalkanes are alkanes which form an internal ring within the H C C molecule. -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -
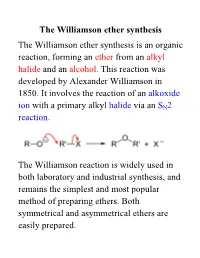
Williamson Ether Synthesis the Williamson Ether Synthesis Is an Organic Reaction, Forming an Ether from an Alkyl Halide and an Alcohol
The Williamson ether synthesis The Williamson ether synthesis is an organic reaction, forming an ether from an alkyl halide and an alcohol. This reaction was developed by Alexander Williamson in 1850. It involves the reaction of an alkoxide ion with a primary alkyl halide via an SN2 reaction. The Williamson reaction is widely used in both laboratory and industrial synthesis, and remains the simplest and most popular method of preparing ethers. Both symmetrical and asymmetrical ethers are easily prepared. The reaction for this week: an example of a Williamson ether synthesis acetaminophen ethyl iodide phenacetin starting material reagent product Phenacetin may be synthesized as an example of the Williamson ether synthesis The first synthesis of phenacetin was reported in 1878 by Harmon Morse. Procedure 1. Weigh an Extra-Strength Tylenol tablet. Pulverize the tablet with mortar and pestle. Weigh out 0.22 g and place it in a dry 15-ml round-bottom flask along with 0.28 g of finely pulverized K2CO3 (mortar and pestle) and 3.0 mL of butanone. Carefully add 0.28 mL of ethyl iodide with a syringe. 2. Add a stir bar; attach a microscale water-cooled condenser to the flask. Heat the mixture under reflux directly on a hot plate at medium setting for 1 hour. In the meantime, obtain the IR of acetaminophen. 3. Turn off the heat. Allow the mixture to cool down. Add 4 mL of water to the flask and transfer its contents to a 16 x 125 mm test tube with a screw cap. Rinse round-bottom flask 4 times with 1 mL of tert-butyl methyl ether (BME) and add the rinsings to the test tube.