On Stream Ecosystem Functioning and Structure in Cape Breton Highlands, Nova Scotia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Populations, Movements and Seasonal Distribution of Mergansers
F y F Environment Canada Environnement Canada Wildlife Service Service de la Faune Population s9 movements and seasonal distribution of mergansers in northern Cape Breton Island by A. J. Erskine Canadian Wildlife Service Report Series-Number 17 Issued under the authority of the Honourable Jack Davis, P.C., M.P. Minister of the Environment John S. Tener, Director Canadian Wildlife Service ©Crown Copyrights reserved Available by mail from Information Canada, Ottawa, and at the following Information Canada bookshops: Halifax 1735 Barrington Street Montreal 1182 St. Catherine Street West Ottawa 171 Slater Street Toronto 221 Yonge Street Winnipeg 393 Portage Avenue Vancouver 657 Granville Street or through your bookseller Price $1.00 Catalogue No. CW65-8/17 Price subject to change without notice Information Canada, Ottawa, 1972 Design: Gottschalk+Ash Ltd. Cover photo by Norman R. Lightfoot Contents 7 Acknowledgements 8 Perspective 9 Abstract 9 Resume 11 Introduction 13 The study area 15 Methods 15 Assessment of merganser populations 17 Banding and associated studies 17 Results 17 Seasonal chronology within study area 20 Movements of mergansers shoivn by band recoveries 22 Merganser populations in northern Cape Breton Island 22 The Margaree River system 25 Other river systems and Lake Ainslie 26 Discussion 26 Seasonal chronology 28 Movements shown by band recoveries 28 Merganser populations 28 77ie Margaree River system 30 Other river systems and Lake Ainslie 31 The aftermath of merganser shooting 32 Literature cited 33 Appendices 5 List of tables List of appendices List of figures 1. Numbers of recoveries in 1957-69, by 1. Winter counts of mergansers, 1960-64, 1. -

American Eel Anguilla Rostrata
COSEWIC Assessment and Status Report on the American Eel Anguilla rostrata in Canada SPECIAL CONCERN 2006 COSEWIC COSEPAC COMMITTEE ON THE STATUS OF COMITÉ SUR LA SITUATION ENDANGERED WILDLIFE DES ESPÈCES EN PÉRIL IN CANADA AU CANADA COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC 2006. COSEWIC assessment and status report on the American eel Anguilla rostrata in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. x + 71 pp. (www.sararegistry.gc.ca/status/status_e.cfm). Production note: COSEWIC would like to acknowledge V. Tremblay, D.K. Cairns, F. Caron, J.M. Casselman, and N.E. Mandrak for writing the status report on the American eel Anguilla rostrata in Canada, overseen and edited by Robert Campbell, Co-chair (Freshwater Fishes) COSEWIC Freshwater Fishes Species Specialist Subcommittee. Funding for this report was provided by Environment Canada. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: (819) 997-4991 / (819) 953-3215 Fax: (819) 994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Évaluation et Rapport de situation du COSEPAC sur l’anguille d'Amérique (Anguilla rostrata) au Canada. Cover illustration: American eel — (Lesueur 1817). From Scott and Crossman (1973) by permission. ©Her Majesty the Queen in Right of Canada 2004 Catalogue No. CW69-14/458-2006E-PDF ISBN 0-662-43225-8 Recycled paper COSEWIC Assessment Summary Assessment Summary – April 2006 Common name American eel Scientific name Anguilla rostrata Status Special Concern Reason for designation Indicators of the status of the total Canadian component of this species are not available. -

Atlantic Salmon Stock Status on Rivers in the Northumberland Strait, Nova
Department of Fisheries and Oceans Ministère des pêches et océan s Canadian Stock Assessment Secretariat Secrétariat canadien pour l'évaluation des -stocks Research Document 97/22 Document de recherche 97/22 Not to be cited without Ne pas citer sans permission of the authors ' autorisation des auteurs ' Atlantic salmon (Salmo salar L .) stock status on rivers in the Northumberland Strait, Nova Scotia area, in 1996 S. F. O'Neil, D. A. Longard, and C . J. Harvie Diadromous Fish Division Science Branch Maritimes Region P.O.Box 550 Halifax, N .S. B3J 2S7 ' This series documents the scientific basis for ' La présente série documente les bases the evaluation of fisheries resources in Canada . scientifiques des évaluations des ressources As such, it addresses the issues of the day in halieutiques du Canada. Elle traite des the time frames required and the documents it problèmes courants selon les échéanciers contains are not intended as definitive dictés. Les documents qu'elle contient ne statements on the subjects addressed but doivent pas être considérés comme des rather as progress reports on ongoing énoncés définitifs sur les sujets traités, mais investigations . plutôt comme des rapports d'étape sur les études en cours. Research documents are produced in the Les documents de recherche sont publiés dans official language in which they are provided to la langue officielle utilisée dans le manuscrit the Secretariat . envoyé au secrétariat . ii Abstrac t Fifteen separate rivers on the Northumberland Strait shore of Nova Scotia support Atlantic salmon stocks. Stock status information for 1996 is provided for nine of those stocks based on the conservation requirements and escapements calculated either from mark-and-recapture experiments (East River, Pictou and River Philip) or capture (exploitation) rates in the angling fishery. -

Humber River, Newfoundland, 199 5
Not to be cited without Ne pas citer sans permission of the authors' autorisation des auteurs ' DFO Atlantic Fisheries MPO Pêches de l'Atlantique Research Document 96/139 Document de recherche 961139 THE STATUS OF THE ATLANTIC SALMON STOCK OF THE HUMBER RIVER, NEWFOUNDLAND, 199 5 C.C. Mullins and D .G. Reddin Department of Fisheries and Ocean s Science Branch 1Regent Square Corner Brook, Newfoundland A2H 7K6 'This series documents the scientific basis for the 'La présente série documente les bases scientifiques des evaluation of fisheries resources in Atlantic Canada . As évaluations des ressources halieutiques sur la côte such, it addresses the issues of the day in the time frames atlantique du Canada . Elle traite des problèmes courants required and the documents it contains are not intended selon les échéanciers dictés . Les documents qu'elle as definitive statements on the subjects addressed but contient ne doivent pas être considérés comme des rather as progress reports on ongoing investigations. énoncés définitifs sur les sujets traités, mais plutôt comme des rapports d'étape sur les études en cours . Research documents are produced in the official language Les Documents de recherche sont publiés dans la langue in which they are provided to the secretariat. officielle utilisée dans le manuscrit envoyé au secrétariat. 1 ABSTRACT This is the sixth assessment of the Atlantic salmon stock of the Humber River . Indices of abundance are mark and recapture estimates of run size, angling catch and effort data and public consultations . Returns of small salmon in 1995 were the highest and large salmon were the second highest in six years of assessment which includesd two pre- moratoium years (1990 and 1991) . -

Geology of the West-Central Cape Breton Highlands, Nova Scotia
Geological Survey of Canada Commission géologique du Canada Paper 87-13 GEOLOGY OF THE WEST-CENTRAL CAPE BRETON HIGHLANDS, NOVA SCOTIA R.A. Jamieson P. Tall man J.A. Marcotte H.E. Plint K.A. Connors 1987 Canada Department of Mines and Energy Contribution to Canada-Nova Scotia Mineral Development Agreement 1984-89, a subsidiary agreement under the Economic and Regional Development Agreement. Project funded by the Geological Survey of Canada. Contribution à l'Entente auxiliaire Canada/ Nouvelle Ecosse sur l'Exploitation minérale 1984-89 faisant partie de l'Entente de développement économique et régional. Ce projet a été financé par la Commission géologique du Canada. Energy, Mines and Énergie, Mines et Resources Canada Resso1 Tces Canada Geological Survey of Canada Paper 87-13 GEOLOGY OF THE WEST-CENTRAL CAPE BRETON HIGHLANDS, NOVA SCOTIA R.A. Jamieson P. Tallman J.A. Marcotte H.E. Plint K.A. Connors 1987 GEOLOGY OF THE WEST-CENTRAL CAPE BRETON HIGHLANDS, NOVA SCOTIA Abstract Mapping of metavolcanic, metasedimentary, and metaplutonic rocks in the vicinity of the Northeast Margaree River, west-central Cape Breton Highlands, has been carried out on 1:10 000 and 1:25 000 scales. The Jumping Brook metamorphic suite (redefined) consists of a lower unit of metabasalts and associated pyroclastic and sedimentary rocks (Faribault Brook metavolcanics), overlain by quartz-rich sedimentary rocks with local conglomerate and tuff (Barren Brook schist), and semi-pelitic to psammitic schists (Dauphinee Brook schist), which grade into porphyroblastic schists ranging up to staurolite-kyanite grade (Corney Brook schist). Medium grained amphibolite (George Brook amphibolite) with relict dioritic texture is common in the sequence and probably represents synvolcanic intrusions. -

Status of Atlantic Salmon (Salmo Salar L.) Stocks in Rivers of Nova Scotia Flowing Into the Gulf of St
C S A S S C C S Canadian Science Advisory Secretariat Secrétariat canadien de consultation scientifique Research Document 2012/147 Document de recherche 2012/147 Gulf Region Région du Golfe Status of Atlantic salmon (Salmo salar Etat des populations de saumon L.) stocks in rivers of Nova Scotia atlantique (Salmo salar) dans les rivières flowing into the Gulf of St. Lawrence de la Nouvelle-Ecosse qui déversent (SFA 18) dans le golfe du Saint-Laurent (ZPS 18) C. Breau Fisheries and Oceans Canada / Pêches et Océans Canada Gulf Fisheries Centre / Centre des Pêches du Golfe 343 University Avenue / 343 avenue de l’Université P.O. Box / C.P. 5030 Moncton, NB / N.-B. E1C 9B6 This series documents the scientific basis for the La présente série documente les fondements evaluation of aquatic resources and ecosystems scientifiques des évaluations des ressources et des in Canada. As such, it addresses the issues of écosystèmes aquatiques du Canada. Elle traite des the day in the time frames required and the problèmes courants selon les échéanciers dictés. documents it contains are not intended as Les documents qu’elle contient ne doivent pas être definitive statements on the subjects addressed considérés comme des énoncés définitifs sur les but rather as progress reports on ongoing sujets traités, mais plutôt comme des rapports investigations. d’étape sur les études en cours. Research documents are produced in the official Les documents de recherche sont publiés dans la language in which they are provided to the langue officielle utilisée dans le manuscrit envoyé au Secretariat. Secrétariat. This document is available on the Internet at Ce document est disponible sur l’Internet à www.dfo-mpo.gc.ca/csas-sccs ISSN 1499-3848 (Printed / Imprimé) ISSN 1919-5044 (Online / En ligne) © Her Majesty the Queen in Right of Canada, 2013 © Sa Majesté la Reine du Chef du Canada, 2013 TABLE OF CONTENTS ABSTRACT................................................................................................................................ -
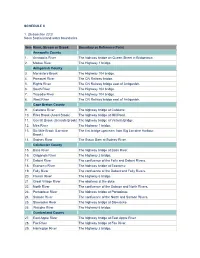
Nova Scotia Inland Water Boundaries Item River, Stream Or Brook
SCHEDULE II 1. (Subsection 2(1)) Nova Scotia inland water boundaries Item River, Stream or Brook Boundary or Reference Point Annapolis County 1. Annapolis River The highway bridge on Queen Street in Bridgetown. 2. Moose River The Highway 1 bridge. Antigonish County 3. Monastery Brook The Highway 104 bridge. 4. Pomquet River The CN Railway bridge. 5. Rights River The CN Railway bridge east of Antigonish. 6. South River The Highway 104 bridge. 7. Tracadie River The Highway 104 bridge. 8. West River The CN Railway bridge east of Antigonish. Cape Breton County 9. Catalone River The highway bridge at Catalone. 10. Fifes Brook (Aconi Brook) The highway bridge at Mill Pond. 11. Gerratt Brook (Gerards Brook) The highway bridge at Victoria Bridge. 12. Mira River The Highway 1 bridge. 13. Six Mile Brook (Lorraine The first bridge upstream from Big Lorraine Harbour. Brook) 14. Sydney River The Sysco Dam at Sydney River. Colchester County 15. Bass River The highway bridge at Bass River. 16. Chiganois River The Highway 2 bridge. 17. Debert River The confluence of the Folly and Debert Rivers. 18. Economy River The highway bridge at Economy. 19. Folly River The confluence of the Debert and Folly Rivers. 20. French River The Highway 6 bridge. 21. Great Village River The aboiteau at the dyke. 22. North River The confluence of the Salmon and North Rivers. 23. Portapique River The highway bridge at Portapique. 24. Salmon River The confluence of the North and Salmon Rivers. 25. Stewiacke River The highway bridge at Stewiacke. 26. Waughs River The Highway 6 bridge. -

South Western Nova Scotia
Netukulimk of Aquatic Natural Life “The N.C.N.S. Netukulimkewe’l Commission is the Natural Life Management Authority for the Large Community of Mi’kmaq /Aboriginal Peoples who continue to reside on Traditional Mi’Kmaq Territory in Nova Scotia undisplaced to Indian Act Reserves” P.O. Box 1320, Truro, N.S., B2N 5N2 Tel: 902-895-7050 Toll Free: 1-877-565-1752 2 Netukulimk of Aquatic Natural Life N.C.N.S. Netukulimkewe’l Commission Table of Contents: Page(s) The 1986 Proclamation by our late Mi’kmaq Grand Chief 4 The 1994 Commendation to all A.T.R.A. Netukli’tite’wk (Harvesters) 5 A Message From the N.C.N.S. Netukulimkewe’l Commission 6 Our Collective Rights Proclamation 7 A.T.R.A. Netukli’tite’wk (Harvester) Duties and Responsibilities 8-12 SCHEDULE I Responsible Netukulimkewe’l (Harvesting) Methods and Equipment 16 Dangers of Illegal Harvesting- Enjoy Safe Shellfish 17-19 Anglers Guide to Fishes Of Nova Scotia 20-21 SCHEDULE II Specific Species Exceptions 22 Mntmu’k, Saqskale’s, E’s and Nkata’laq (Oysters, Scallops, Clams and Mussels) 22 Maqtewe’kji’ka’w (Small Mouth Black Bass) 23 Elapaqnte’mat Ji’ka’w (Striped Bass) 24 Atoqwa’su (Trout), all types 25 Landlocked Plamu (Landlocked Salmon) 26 WenjiWape’k Mime’j (Atlantic Whitefish) 26 Lake Whitefish 26 Jakej (Lobster) 27 Other Species 33 Atlantic Plamu (Salmon) 34 Atlantic Plamu (Salmon) Netukulimk (Harvest) Zones, Seasons and Recommended Netukulimk (Harvest) Amounts: 55 SCHEDULE III Winter Lake Netukulimkewe’l (Harvesting) 56-62 Fishing and Water Safety 63 Protecting Our Community’s Aboriginal and Treaty Rights-Community 66-70 Dispositions and Appeals Regional Netukulimkewe’l Advisory Councils (R.N.A.C.’s) 74-75 Description of the 2018 N.C.N.S. -

Recovery Potential Assessment for Eastern Cape Breton Atlantic Salmon (Salmo Salar): Habitat Requirements and Availability; and Threats to Populations
Canadian Science Advisory Secretariat (CSAS) Research Document 2014/071 Maritimes Region Recovery Potential Assessment for Eastern Cape Breton Atlantic Salmon (Salmo salar): Habitat Requirements and Availability; and Threats to Populations A.J.F. Gibson, T.L. Horsman, J.S. Ford, and E.A. Halfyard Fisheries and Oceans Canada Science Branch, Maritimes Region P.O. Box 1006, Dartmouth, Nova Scotia Canada, B2Y 4A2 December 2014 Foreword This series documents the scientific basis for the evaluation of aquatic resources and ecosystems in Canada. As such, it addresses the issues of the day in the time frames required and the documents it contains are not intended as definitive statements on the subjects addressed but rather as progress reports on ongoing investigations. Research documents are produced in the official language in which they are provided to the Secretariat. Published by: Fisheries and Oceans Canada Canadian Science Advisory Secretariat 200 Kent Street Ottawa ON K1A 0E6 http://www.dfo-mpo.gc.ca/csas-sccs/ [email protected] © Her Majesty the Queen in Right of Canada, 2014 ISSN 1919-5044 Correct citation for this publication: Gibson, A.J.F., Horsman, T., Ford, J. and Halfyard, E.A. 2014. Recovery Potential Assessment for Eastern Cape Breton Atlantic Salmon (Salmo salar): Habitat requirements and availability; and threats to populations. DFO Can. Sci. Advis. Sec. Res. Doc. 2014/071. vii + 141 p. TABLE OF CONTENTS ABSTRACT ................................................................................................................................ -
Cape Breton Highlands National Park
Cape Breton Highlands National Park Nova Scotia Introducing a Park and an Idea Canada covers half a continent, fronts on three oceans, Cape Breton and stretches from the extreme Arctic more than halfway to the equator. There is a great variety of land forms in this immense country, and Canada's national parks have Highlands been created to preserve important examples for you and for generations to come. The National Parks Act of 1930 specifies that national parks are "dedicated to the people . for their benefit, National Park education and enjoyment" and must remain "unimpaired for the enjoyment of future generations." Cape Breton Highlands National Park. 367 square miles in area, forms part of a huge tableland rising over 1,700 Nova Scotia feet above the sea in the northern section of Cape Breton Conducted field trip Island, Nova Scotia. With its rugged Atlantic shoreline rocks of varying sizes), sandstone, and shale. Later, when and mountainous background, the park is reminiscent of parts of the sea evaporated, it left behind reddish beds with the coastal areas of Scotland. gypsum deposits. In the park, the flat-bedded sedimentary rock of the The Park Environment earth's crtist folded, cracked and was upended during the Each national park has its own character, its unique story last 500 million years. Molten granite from below was as a living, outdoor museum. Cape Breton Highlands' forced upward through these cracks or faults, cooled, and story is the drama of a mountainous landscape with bold now forms the hardest rock in the park. headlands, numerous streams, lakes, and forests as well Over the course of millions of years, rivers eroded the as treeless barrens. -

Gaspereau Maritimes Region Overview Limits, Are Implemented
Fisheries Pêches and Oceans et Océans DFO Science Maritimes Region Stock Status Report D3-17 Species proportion Blueback herring Alewife Bon Harriott Leim & Scott 1966 Gaspereau Southern Gulf Maritimes Region Overview Background Bay of Fundy Alewife (Alosa pseudoharengus) and blueback herring Nova Scotia Coast (Alosa aestivalis) are anadromous clupeids that frequent the rivers of the Maritimes. They are collectively referred to as gaspereau. Blueback herring occur in fewer rivers and are generally less abundant than alewives where both species co-occur. Spawning migrations of alewives typically begin in late April or early May, depending upon The Fishery geographic area and water temperature, peak in late May or early June and are completed by late June or early Landings (t) July. Blueback herring enter the river about 2 weeks later Year 70-79 80-89 1992 1993 1994 1995 1996 than do alewives. Both species return to sea soon after Avg. Avg. spawning. Young-of-the-year gaspereau spend, at most, S.Gulf 3704 4848 4544 4722 3806 3452 2150 the first summer and fall in fresh water before migrating NS.Coast 1279 893 497 803 973 1439 1365 to the sea. Both species recruit to the spawning stock over B. of F. 4184 1836 1618 1137 863 1230 1275 2-4 years. Spawning occurs first in both species at age 3 and virtually all fish have spawned by age 6. The mean TOTAL 9167 7578 6659 6662 5642 6120 4790 age at first spawning is usually older for females than for males. Repeat spawners may form a high proportion (35- 90%) of the stocks of both species, with higher The gaspereau fisheries are regulated by proportions of repeat spawners where exploitation is low. -

Biological Characteristics of Sea-Run Salmonids from a Spring Angler Creel Survey on Five Northumberland Strait River Systems In
Biological characteristics of sea-run salmonids from a spring angler creel survey on five Northumberland Strait river systems in Nova Scotia, and management implications. By John MacMillan and Jason LeBlanc Spring 2002 Inland Fisheries Division Nova Scotia Department of Agriculture and Fisheries Box 700 Pictou Nova Scotia B0K 1H0 Acknowledgements We thank Shane O’Neil, Fisheries Biologist, Fisheries and Oceans Canada, for the critical review of this paper. Al McNeill, Ralph Heighton, Darryl Murrant, Bob Bancroft, Veronica Brandt, Struan MacIntosh, John MacGillvary, Frank MacDonald, Penny Spears, and Jack MacNeil participated in the field component of this survey. Scale analysis and aging of trout was conducted by Ralph Heighton and Al McNeill. We acknowledge Don MacLean and Murray Hill for providing resources for this survey and, as well as, Carla MacMillan, for providing a useful edit. i Table of Contents Acknowledgements .............................................................................. i List of Tables ...................................................................................... iii List of Figures ..................................................................................... iii List of Appendicies ............................................................................ iii Abstract .............................................................................................. 1 Introduction ......................................................................................... 2 Methods .............................................................................................