HSF1 Antibody (N-Term) Affinity Purified Rabbit Polyclonal Antibody (Pab) Catalog # Ap14189a
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CREST Monoclonal ANTIBODY Catalog Number:60314-1-Ig
For Research Use Only CREST Monoclonal ANTIBODY www.ptglab.com Catalog Number:60314-1-Ig Catalog Number: GenBank Accession Number: Purification Method: Basic Information 60314-1-Ig BC034494 Protein G purification Size: GeneID (NCBI): CloneNo.: 150UL , Concentration: 1000 μg/ml by 26039 3D5D10 Bradford method using BSA as the Full Name: standard; synovial sarcoma translocation gene Source: on chromosome 18-like 1 Mouse Calculated MW: Isotype: 396 aa, 43 kDa IgG1 Observed MW: Immunogen Catalog Number: 50 kDa AG3119 Tested Applications: Applications WB, ELISA Species Specificity: human, rat, mouse SS18-like 1(SS18L1) is a transcriptional activator that is required for calcium-dependent dendritic growth and Background Information branching in cortical neurons. It's also a nuclear protein interacts with CREB-binding protein and expressed in the developing brain. It helps regulate neuronal morphogenesis in calcuim-dependent manner. The N-terminal domain of SS18L1 is required for suppressing transactivation in the basal state, while the C-terminal domain is required for calcium-induced transactivation. It's part of the CREST-BRG1 complex, a multiprotein complex that regulates promoter activation by orchestrating a calcium-dependent release of a repressor complex and a recruitment of an activator complex. This complex also binds to the NR2B promoter, and activity-dependent induction of NR2B expression involves a release of HDAC1 and recruitment of CREBBP. The calculated molecular weight of CREST is about 43 kDa, but the modified of CREST protein is 55 kDa (PMID: 25888396). Storage: Storage Store at -20°C. Stable for one year after shipment. Storage Buffer: PBS with 0.02% sodium azide and 50% glycerol pH 7.3. -

HSF1 Polyclonal Antibody Catalog # AP70419
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 HSF1 Polyclonal Antibody Catalog # AP70419 Specification HSF1 Polyclonal Antibody - Product Information Application WB Primary Accession Q00613 Reactivity Human, Mouse Host Rabbit Clonality Polyclonal HSF1 Polyclonal Antibody - Additional Information Gene ID 3297 Other Names HSF1; HSTF1; Heat shock factor protein 1; HSF 1; Heat shock transcription factor 1; HSTF 1 Dilution WB~~Western Blot: 1/500 - 1/2000. Immunohistochemistry: 1/100 - 1/300. HSF1 Polyclonal Antibody - Background Immunofluorescence: 1/200 - 1/1000. ELISA: 1/10000. Not yet tested in other Function as a stress-inducible and applications. DNA-binding transcription factor that plays a central role in the transcriptional activation of Format the heat shock response (HSR), leading to the Liquid in PBS containing 50% glycerol, 0.5% expression of a large class of molecular BSA and 0.02% sodium azide. chaperones heat shock proteins (HSPs) that protect cells from cellular insults' damage Storage Conditions -20℃ (PubMed:1871105, PubMed:11447121, PubMed:1986252, PubMed:7760831, PubMed:7623826, PubMed:8946918, PubMed:8940068, PubMed:9341107, HSF1 Polyclonal Antibody - Protein Information PubMed:9121459, PubMed:9727490, PubMed:9499401, PubMed:9535852, Name HSF1 (HGNC:5224) PubMed:12659875, PubMed:12917326, PubMed:15016915, PubMed:25963659, Synonyms HSTF1 PubMed:26754925). In unstressed cells, is present in a HSP90-containing multichaperone Function complex that maintains it in a non-DNA-binding Functions -

A Novel FISH Assay for SS18–SSX Fusion Type in Synovial Sarcoma
Laboratory Investigation (2004) 84, 1185–1192 & 2004 USCAP, Inc All rights reserved 0023-6837/04 $30.00 www.laboratoryinvestigation.org A novel FISH assay for SS18–SSX fusion type in synovial sarcoma Cecilia Surace1,2, Ioannis Panagopoulos1, Eva Pa˚lsson1, Mariano Rocchi2, Nils Mandahl1 and Fredrik Mertens1 1Department of Clinical Genetics, Lund University Hospital, Lund, Sweden and 2DAPEG, Section of Genetics, University of Bari, Bari, Italy Synovial sarcoma is a morphologically, clinically and genetically distinct entity that accounts for 5–10% of all soft tissue sarcomas. The t(X;18)(p11.2;q11.2) is the cytogenetic hallmark of synovial sarcoma and is present in more than 90% of the cases. It produces three types of fusion gene formed in part by SS18 from chromosome 18 and by SSX1, SSX2 or, rarely, SSX4 from the X chromosome. The SS18–SSX fusions do not seem to occur in other tumor types, and it has been shown that in synovial sarcoma a clear correlation exists between the type of fusion gene and histologic subtype and, more importantly, clinical outcome. Previous analyses regarding the type of fusion genes have been based on PCR amplification of the fusion transcript, requiring access to good- quality RNA. In order to obtain an alternative tool to diagnose and follow this malignancy, we developed a fluorescence in situ hybridization (FISH) assay that could distinguish between the two most common fusion genes, that is, SS18–SSX1 and SS18–SSX2. The specificity of the selected bacterial artificial chromosome clones used in the detection of these fusion genes, as well as the sensitivity of the analysis in metaphase and interphase cells, was examined in a series of 28 synovial sarcoma samples with known fusion gene status. -

(SS18L1) (NM 198935) Human Tagged ORF Clone Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for RC212373 SYT homolog 1 (SS18L1) (NM_198935) Human Tagged ORF Clone Product data: Product Type: Expression Plasmids Product Name: SYT homolog 1 (SS18L1) (NM_198935) Human Tagged ORF Clone Tag: Myc-DDK Symbol: SS18L1 Synonyms: CREST; LP2261 Vector: pCMV6-Entry (PS100001) E. coli Selection: Kanamycin (25 ug/mL) Cell Selection: Neomycin ORF Nucleotide >RC212373 representing NM_198935 Sequence: Red=Cloning site Blue=ORF Green=Tags(s) TTTTGTAATACGACTCACTATAGGGCGGCCGGGAATTCGTCGACTGGATCCGGTACCGAGGAGATCTGCC GCCGCGATCGCC ATGTCCGTGGCCTTCGCGTCTGCCCGGCCAAGAGGCAAAGGGGAGGTTACGCAGCAAACCATCCAGAAGA TGCTGGACGAGAACCACCACCTGATCCAGTGCATCCTGGAGTACCAGAGCAAGGGCAAGACGGCCGAGTG CACGCAGTACCAGCAGATCCTGCACCGGAACCTGGTATACCTGGCCACGATCGCAGACTCCAACCAGAAC ATGCAGTCCCTGCTTCCTGCCCCGCCCACGCAGAACATGAACCTGGGCCCTGGAGCCCTGACTCAGAGCG GCTCCAGCCAGGGCCTGCACTCTCAGGGCAGCCTGAGTGACGCCATCAGCACGGGCCTGCCACCCTCCTC CCTCCTGCAGGGCCAGATTGGCAACGGGCCGAGCCACGTGTCCATGCAGCAGACGGCGCCTAACACGCTG CCCACCACCTCCATGAGCATCTCTGGGCCCGGCTACAGCCACGCGGGACCCGCCTCGCAGGGCGTCCCCA TGCAGGGGCAAGGCACCATCGGCAACTACGTGTCTCGGACCAACATCAACATGCAGTCCAACCCAGTCTC CATGATACAGCAGCAGGCGGCCACGTCGCACTACAGCTCGGCGCAGGGCGGCAGCCAGCACTACCAGGGC CAGTCGTCCATCGCCATGATGGGGCAGGGCAGCCAGGGGAGCAGCATGATGGGGCAGCGGCCCATGGCGC CCTACCGGCCCTCCCAGCAAGGCTCTTCCCAGCAGTACCTGGGCCAGGAGGAGTACTATGGCGAGCAGTA CAGCCACAGCCAGGGCGCCGCGGAGCCCATGGGCCAGCAGTACTACCCCGACGGCCATGGCGATTACGCC -

HSF1 Antibody Purified Mouse Monoclonal Antibody Catalog # Ao1966a
10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 HSF1 Antibody Purified Mouse Monoclonal Antibody Catalog # AO1966a Specification HSF1 Antibody - Product Information Application E, WB, IF, IHC Primary Accession Q00613 Reactivity Human Host Mouse Clonality Monoclonal Isotype IgG2b Calculated MW 57.3kDa KDa Description The product of this gene is a heat-shock transcription factor. Transcription of heat-shock genes is rapidly induced after temperature stress. Hsp90, by itself and/or associated with multichaperone complexes, is a major repressor of this gene. Immunogen Purified recombinant fragment of human HSF1 (AA: 256-359) expressed in E. Coli. Formulation Purified antibody in PBS with 0.05% sodium azide. HSF1 Antibody - Additional Information Gene ID 3297 Other Names Heat shock factor protein 1, HSF 1, Heat shock transcription factor 1, HSTF 1, HSF1, HSTF1 Dilution E~~1/10000 WB~~1/500 - 1/2000 IF~~1/200 - 1/1000 IHC~~1/200 - 1/1000 Storage Maintain refrigerated at 2-8°C for up to 6 months. For long term storage store at -20°C in small aliquots to prevent freeze-thaw cycles. Precautions HSF1 Antibody is for research use only and not for use in diagnostic or therapeutic procedures. HSF1 Antibody - Protein Information Name HSF1 (HGNC:5224) Page 1/3 10320 Camino Santa Fe, Suite G San Diego, CA 92121 Tel: 858.875.1900 Fax: 858.622.0609 Synonyms HSTF1 Function Functions as a stress-inducible and DNA-binding transcription factor that plays a central role in the transcriptional activation of the heat shock -

Intrinsic Disorder of the BAF Complex: Roles in Chromatin Remodeling and Disease Development
International Journal of Molecular Sciences Article Intrinsic Disorder of the BAF Complex: Roles in Chromatin Remodeling and Disease Development Nashwa El Hadidy 1 and Vladimir N. Uversky 1,2,* 1 Department of Molecular Medicine, Morsani College of Medicine, University of South Florida, 12901 Bruce B. Downs Blvd. MDC07, Tampa, FL 33612, USA; [email protected] 2 Laboratory of New Methods in Biology, Institute for Biological Instrumentation of the Russian Academy of Sciences, Federal Research Center “Pushchino Scientific Center for Biological Research of the Russian Academy of Sciences”, Pushchino, 142290 Moscow Region, Russia * Correspondence: [email protected]; Tel.: +1-813-974-5816; Fax: +1-813-974-7357 Received: 20 September 2019; Accepted: 21 October 2019; Published: 23 October 2019 Abstract: The two-meter-long DNA is compressed into chromatin in the nucleus of every cell, which serves as a significant barrier to transcription. Therefore, for processes such as replication and transcription to occur, the highly compacted chromatin must be relaxed, and the processes required for chromatin reorganization for the aim of replication or transcription are controlled by ATP-dependent nucleosome remodelers. One of the most highly studied remodelers of this kind is the BRG1- or BRM-associated factor complex (BAF complex, also known as SWItch/sucrose non-fermentable (SWI/SNF) complex), which is crucial for the regulation of gene expression and differentiation in eukaryotes. Chromatin remodeling complex BAF is characterized by a highly polymorphic structure, containing from four to 17 subunits encoded by 29 genes. The aim of this paper is to provide an overview of the role of BAF complex in chromatin remodeling and also to use literature mining and a set of computational and bioinformatics tools to analyze structural properties, intrinsic disorder predisposition, and functionalities of its subunits, along with the description of the relations of different BAF complex subunits to the pathogenesis of various human diseases. -

Mouse Ss18l1 Conditional Knockout Project (CRISPR/Cas9)
https://www.alphaknockout.com Mouse Ss18l1 Conditional Knockout Project (CRISPR/Cas9) Objective: To create a Ss18l1 conditional knockout Mouse model (C57BL/6J) by CRISPR/Cas-mediated genome engineering. Strategy summary: The Ss18l1 gene (NCBI Reference Sequence: NM_178750 ; Ensembl: ENSMUSG00000039086 ) is located on Mouse chromosome 2. 11 exons are identified, with the ATG start codon in exon 1 and the TAA stop codon in exon 11 (Transcript: ENSMUST00000041126). Exon 2 will be selected as conditional knockout region (cKO region). Deletion of this region should result in the loss of function of the Mouse Ss18l1 gene. To engineer the targeting vector, homologous arms and cKO region will be generated by PCR using BAC clone RP23-343C13 as template. Cas9, gRNA and targeting vector will be co-injected into fertilized eggs for cKO Mouse production. The pups will be genotyped by PCR followed by sequencing analysis. Note: Mice homozygous for disruptions in this gene have central nervous system and coordination defects. They grow slowly and usually die before adulthood. Those that do survive are infertile. Exon 2 starts from about 5.8% of the coding region. The knockout of Exon 2 will result in frameshift of the gene. The size of intron 1 for 5'-loxP site insertion: 9595 bp, and the size of intron 2 for 3'-loxP site insertion: 768 bp. The size of effective cKO region: ~577 bp. The cKO region does not have any other known gene. Page 1 of 8 https://www.alphaknockout.com Overview of the Targeting Strategy Wildtype allele gRNA region 5' gRNA region 3' 1 2 3 11 Targeting vector Targeted allele Constitutive KO allele (After Cre recombination) Legends Exon of mouse Ss18l1 Homology arm cKO region loxP site Page 2 of 8 https://www.alphaknockout.com Overview of the Dot Plot Window size: 10 bp Forward Reverse Complement Sequence 12 Note: The sequence of homologous arms and cKO region is aligned with itself to determine if there are tandem repeats. -

The DNA Sequence and Comparative Analysis of Human Chromosome 20
articles The DNA sequence and comparative analysis of human chromosome 20 P. Deloukas, L. H. Matthews, J. Ashurst, J. Burton, J. G. R. Gilbert, M. Jones, G. Stavrides, J. P. Almeida, A. K. Babbage, C. L. Bagguley, J. Bailey, K. F. Barlow, K. N. Bates, L. M. Beard, D. M. Beare, O. P. Beasley, C. P. Bird, S. E. Blakey, A. M. Bridgeman, A. J. Brown, D. Buck, W. Burrill, A. P. Butler, C. Carder, N. P. Carter, J. C. Chapman, M. Clamp, G. Clark, L. N. Clark, S. Y. Clark, C. M. Clee, S. Clegg, V. E. Cobley, R. E. Collier, R. Connor, N. R. Corby, A. Coulson, G. J. Coville, R. Deadman, P. Dhami, M. Dunn, A. G. Ellington, J. A. Frankland, A. Fraser, L. French, P. Garner, D. V. Grafham, C. Grif®ths, M. N. D. Grif®ths, R. Gwilliam, R. E. Hall, S. Hammond, J. L. Harley, P. D. Heath, S. Ho, J. L. Holden, P. J. Howden, E. Huckle, A. R. Hunt, S. E. Hunt, K. Jekosch, C. M. Johnson, D. Johnson, M. P. Kay, A. M. Kimberley, A. King, A. Knights, G. K. Laird, S. Lawlor, M. H. Lehvaslaiho, M. Leversha, C. Lloyd, D. M. Lloyd, J. D. Lovell, V. L. Marsh, S. L. Martin, L. J. McConnachie, K. McLay, A. A. McMurray, S. Milne, D. Mistry, M. J. F. Moore, J. C. Mullikin, T. Nickerson, K. Oliver, A. Parker, R. Patel, T. A. V. Pearce, A. I. Peck, B. J. C. T. Phillimore, S. R. Prathalingam, R. W. Plumb, H. Ramsay, C. M. -

Functional Annotation of the Human Retinal Pigment Epithelium
BMC Genomics BioMed Central Research article Open Access Functional annotation of the human retinal pigment epithelium transcriptome Judith C Booij1, Simone van Soest1, Sigrid MA Swagemakers2,3, Anke HW Essing1, Annemieke JMH Verkerk2, Peter J van der Spek2, Theo GMF Gorgels1 and Arthur AB Bergen*1,4 Address: 1Department of Molecular Ophthalmogenetics, Netherlands Institute for Neuroscience (NIN), an institute of the Royal Netherlands Academy of Arts and Sciences (KNAW), Meibergdreef 47, 1105 BA Amsterdam, the Netherlands (NL), 2Department of Bioinformatics, Erasmus Medical Center, 3015 GE Rotterdam, the Netherlands, 3Department of Genetics, Erasmus Medical Center, 3015 GE Rotterdam, the Netherlands and 4Department of Clinical Genetics, Academic Medical Centre Amsterdam, the Netherlands Email: Judith C Booij - [email protected]; Simone van Soest - [email protected]; Sigrid MA Swagemakers - [email protected]; Anke HW Essing - [email protected]; Annemieke JMH Verkerk - [email protected]; Peter J van der Spek - [email protected]; Theo GMF Gorgels - [email protected]; Arthur AB Bergen* - [email protected] * Corresponding author Published: 20 April 2009 Received: 10 July 2008 Accepted: 20 April 2009 BMC Genomics 2009, 10:164 doi:10.1186/1471-2164-10-164 This article is available from: http://www.biomedcentral.com/1471-2164/10/164 © 2009 Booij et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -
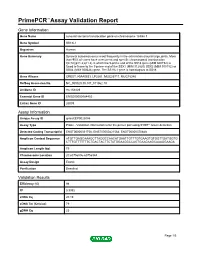
Primepcr™Assay Validation Report
PrimePCR™Assay Validation Report Gene Information Gene Name synovial sarcoma translocation gene on chromosome 18-like 1 Gene Symbol SS18L1 Organism Human Gene Summary Synovial sarcomas occur most frequently in the extremities around large joints. More than 90% of cases have a recurrent and specific chromosomal translocation t(X;18)(p11.2;q11.2) in which the 5-prime end of the SS18 gene (MIM 600192) is fused in-frame to the 3-prime end of the SSX1 (MIM 312820) SSX2 (MIM 300192) or SSX4 (MIM 300326) gene. The SS18L1 gene is homologous to SS18. Gene Aliases CREST, KIAA0693, LP2261, MGC26711, MGC78386 RefSeq Accession No. NC_000020.10, NT_011362.10 UniGene ID Hs.154429 Ensembl Gene ID ENSG00000184402 Entrez Gene ID 26039 Assay Information Unique Assay ID qHsaCEP0025086 Assay Type Probe - Validation information is for the primer pair using SYBR® Green detection Detected Coding Transcript(s) ENST00000331758, ENST00000421564, ENST00000370848 Amplicon Context Sequence ATGTTGAGCAAAGCTTAGGCCAACATGAATTGTTTGTGAAGTGTGGTTGATGGTG CTTTGTTTTTTTCTGACTACTTCTATGGAAGGCCAGTGAAGAAGCAAAGGAAGA Amplicon Length (bp) 79 Chromosome Location 20:60756836-60756944 Assay Design Exonic Purification Desalted Validation Results Efficiency (%) 98 R2 0.9992 cDNA Cq 20.19 cDNA Tm (Celsius) 79 gDNA Cq 25 Page 1/5 PrimePCR™Assay Validation Report Specificity (%) 100 Information to assist with data interpretation is provided at the end of this report. Page 2/5 PrimePCR™Assay Validation Report SS18L1, Human Amplification Plot Amplification of cDNA generated from 25 ng of universal -

Thesis for Word XP
Thesis for doctoral degree (Ph.D.) 2008 Thesis for doctoral degree (Ph.D.) 2008 Molecular Mechanisms Underlying the Oncogenic Function of SS18 and SSX Molecular Mechanisms Underlying the Oncogenic Function of SS18 and SSX Pádraig D’Arcy Fredrik Bredin Pádraig D’Arcy Department of Oncology-Pathology Cancer Center Karolinska Karolinska Institutet, Stockholm, Sweden MOLECULAR MECHANISMS UNDERLYING THE ONCOGENIC FUNCTION OF SS18 AND SSX. Pádraig D'Arcy Stockholm 2008 All previously published papers were reproduced with permission from the publisher. Published by Karolinska Institutet. Printed by Larserics Digital Print AB © Pádraig D'Arcy, 2008 ISBN 978-91-7357-481-5 I’m digging for fire The Pixies To my parents Abstract The SS18 and SSX genes were initially identified based on their reoccurrence as fusion partners in synovial sarcoma. As a result of the specific chromosomal translocation t(X:18), the SS18 gene from chromosome 18 becomes fused with members of the SSX gene family on the X chromosome resulting in the generation of a novel chimeric fusion gene SS18-SSX. The SS18 gene encodes a ubiquitously expressed transcriptional activator, whereas the SSX gene encodes a transcriptional repressor whose expression is restricted to germ cells and numerous cancers. Thus, the resultant SS18-SSX fusion gene encodes a transcription factor with dual trans activation and repression properties; the expression of which is the initiating event of synovial sarcoma. We present the findings that SSX, along with several other members of the CT-antigen family is expressed in mesenchymal stem cells and their expression is down regulated following differentiation. Knockdown of SSX could effectively impair cell migration, a phenotype associated with down regulation of MMP2 expression adding a functional role for SSX in stem and tumor cell migration. -

A Map of Mobile DNA Insertions in the NCI-60 Human Cancer Cell Panel John G
Zampella et al. Mobile DNA (2016) 7:20 DOI 10.1186/s13100-016-0078-4 RESEARCH Open Access A map of mobile DNA insertions in the NCI-60 human cancer cell panel John G. Zampella1, Nemanja Rodić2, Wan Rou Yang2, Cheng Ran Lisa Huang3, Jane Welch3, Veena P. Gnanakkan3, Toby C. Cornish2, Jef D. Boeke3,4,6 and Kathleen H. Burns2,3,4,5* Abstract Background: The National Cancer Institute-60 (NCI-60) cell lines are among the most widely used models of human cancer. They provide a platform to integrate DNA sequence information, epigenetic data, RNA and protein expression, and pharmacologic susceptibilities in studies of cancer cell biology. Genome-wide studies of the complete panel have included exome sequencing, karyotyping, and copy number analyses but have not targeted repetitive sequences. Interspersed repeats derived from mobile DNAs are a significant source of heritable genetic variation, and insertions of active elements can occur somatically in malignancy. Method: We used Transposon Insertion Profiling by microarray (TIP-chip) to map Long INterspersed Element-1 (LINE-1, L1) and Alu Short INterspersed Element (SINE) insertions in cancer genes in NCI-60 cells. We focused this discovery effort on annotated Cancer Gene Index loci. Results: We catalogued a total of 749 and 2,100 loci corresponding to candidate LINE-1 and Alu insertion sites, respectively. As expected, these numbers encompass previously known insertions, polymorphisms shared in unrelated tumor cell lines, as well as unique, potentially tumor-specific insertions. We also conducted association analyses relating individual insertions to a variety of cellular phenotypes. Conclusions: These data provide a resource for investigators with interests in specific cancer gene loci or mobile element insertion effects more broadly.