Petition for Determination of Nonregulated Status: Arctic™ Apple (Malus X Domestica) Events GD743 and GS784
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alternative Fermentations
the best of ® ALTERNATIVE FERMENTATIONS Please note all file contents are Copyright © 2021 Battenkill Communications, Inc. All Rights Reserved. This file is for the buyer’s personal use only. It’s unlawful to share or distribute this file to others in any way including e-mailing it, posting it online, or sharing printed copies with others. MAKING MEAD BY BRAD SMITH ead, which is a fermented beverage made from honey, is one of the oldest alcoholic M beverages. Vessels found in China dating back to 7000 B.C. have organic compounds consistent with fermented honey and rice. Mead was the revered “nectar of the gods” in ancient Greece and the “drink of kings” throughout history, though it has faded to obscurity in modern times. For homebrewers, mead is a great addition to complement your other fermented offerings. Many of your guests may have never tasted a good quality mead or melomel (fruit mead), but almost everyone enjoys this sweet beverage. Using some modern methods, mead is also relatively easy and quick to make, and you can use equipment you already have on hand for homebrewing. MODERN MEADMAKING TECHNIQUES When I started homebrewing back in 1987, the fermentation of mead was a very slow process, taking 12 to 18 Photo by Charles A. Parker/Images Plus Parker/Images A. Charles by Photo months for a mead to fully ferment and age. Honey has antibacterial it highlights the flavor of the honey country may have additional variants. properties and is poor in nutrients, varietal itself. In the US, a lot of honey production particularly nitrogen, resulting in a The variety of honey and strength is still done by small, independent very slow fermentation. -

Native Trees of Georgia
1 NATIVE TREES OF GEORGIA By G. Norman Bishop Professor of Forestry George Foster Peabody School of Forestry University of Georgia Currently Named Daniel B. Warnell School of Forest Resources University of Georgia GEORGIA FORESTRY COMMISSION Eleventh Printing - 2001 Revised Edition 2 FOREWARD This manual has been prepared in an effort to give to those interested in the trees of Georgia a means by which they may gain a more intimate knowledge of the tree species. Of about 250 species native to the state, only 92 are described here. These were chosen for their commercial importance, distribution over the state or because of some unusual characteristic. Since the manual is intended primarily for the use of the layman, technical terms have been omitted wherever possible; however, the scientific names of the trees and the families to which they belong, have been included. It might be explained that the species are grouped by families, the name of each occurring at the top of the page over the name of the first member of that family. Also, there is included in the text, a subdivision entitled KEY CHARACTERISTICS, the purpose of which is to give the reader, all in one group, the most outstanding features whereby he may more easily recognize the tree. ACKNOWLEDGEMENTS The author wishes to express his appreciation to the Houghton Mifflin Company, publishers of Sargent’s Manual of the Trees of North America, for permission to use the cuts of all trees appearing in this manual; to B. R. Stogsdill for assistance in arranging the material; to W. -

Variety Description Origin Approximate Ripening Uses
Approximate Variety Description Origin Ripening Uses Yellow Transparent Tart, crisp Imported from Russia by USDA in 1870s Early July All-purpose Lodi Tart, somewhat firm New York, Early 1900s. Montgomery x Transparent. Early July Baking, sauce Pristine Sweet-tart PRI (Purdue Rutgers Illinois) release, 1994. Mid-late July All-purpose Dandee Red Sweet-tart, semi-tender New Ohio variety. An improved PaulaRed type. Early August Eating, cooking Redfree Mildly tart and crunchy PRI release, 1981. Early-mid August Eating Sansa Sweet, crunchy, juicy Japan, 1988. Akane x Gala. Mid August Eating Ginger Gold G. Delicious type, tangier G Delicious seedling found in Virginia, late 1960s. Mid August All-purpose Zestar! Sweet-tart, crunchy, juicy U Minn, 1999. State Fair x MN 1691. Mid August Eating, cooking St Edmund's Pippin Juicy, crisp, rich flavor From Bury St Edmunds, 1870. Mid August Eating, cider Chenango Strawberry Mildly tart, berry flavors 1850s, Chenango County, NY Mid August Eating, cooking Summer Rambo Juicy, tart, aromatic 16th century, Rambure, France. Mid-late August Eating, sauce Honeycrisp Sweet, very crunchy, juicy U Minn, 1991. Unknown parentage. Late Aug.-early Sept. Eating Burgundy Tart, crisp 1974, from NY state Late Aug.-early Sept. All-purpose Blondee Sweet, crunchy, juicy New Ohio apple. Related to Gala. Late Aug.-early Sept. Eating Gala Sweet, crisp New Zealand, 1934. Golden Delicious x Cox Orange. Late Aug.-early Sept. Eating Swiss Gourmet Sweet-tart, juicy Switzerland. Golden x Idared. Late Aug.-early Sept. All-purpose Golden Supreme Sweet, Golden Delcious type Idaho, 1960. Golden Delicious seedling Early September Eating, cooking Pink Pearl Sweet-tart, bright pink flesh California, 1944, developed from Surprise Early September All-purpose Autumn Crisp Juicy, slow to brown Golden Delicious x Monroe. -

Calvados & Other Fruit Spirits
CALVADOS & OTHER FRUIT SPIRITS There are a lot of different types of fruit based spirits all over the world, most are not particularly famous or consumed other than in the producing country itself, except for the Calvados which goes worldwide. France is the producer of this apple brandy that we call Calvados which will be the primary focus of this section. You might also have come across applejack from North America or other versions of fruit spirits based on cherries, raspberries, pears, plums etc. In short you can base a fruit spirit (brandy) on any kind of fruit and most have been tried and many have very, very old traditions within their country of origin. Calvados Calvados is a fruit spirit produced in northern France where grapes are having a hard time to grow but apples and pears thrive. Calvados is an apple distillate (sometimes with a small addition of pear) and is regulated in French law. In contrast to Cognac and Armagnac which only has one appellation each, Calvados actually has three "Appellation d'origine controlee"; AOC Calvados, AOC Calvados Pays d'Auge and AOC Calvados Domfrontais. Let's break it down below. AOC Calvados Either pot still or copper double-column stills are allowed to be used. Must be aged for at least two years on oak. Accounts for around 70% of the total production of Calvados. AOC Calvados Pays d'Auge Pot stills are to be used exclusively. The fermentation process must be at least 6 weeks contributing to its complex flavour. Must be aged for at least two years on oak. -

PRE Evaluation Report for Malus 'Donald Wyman'
PRE Evaluation Report -- Malus 'Donald Wyman' Plant Risk Evaluator -- PRE™ Evaluation Report Malus 'Donald Wyman' -- Illinois 2017 Farm Bill PRE Project PRE Score: 10 -- Accept (low risk of invasiveness) Confidence: 52 / 100 Questions answered: 20 of 20 -- Valid (80% or more questions answered) Privacy: Public Status: Submitted Evaluation Date: July 19, 2017 This PDF was created on June 15, 2018 Page 1/20 PRE Evaluation Report -- Malus 'Donald Wyman' Plant Evaluated Malus 'Donald Wyman' Image by MBOT Page 2/20 PRE Evaluation Report -- Malus 'Donald Wyman' Evaluation Overview A PRE™ screener conducted a literature review for this plant (Malus 'Donald Wyman') in an effort to understand the invasive history, reproductive strategies, and the impact, if any, on the region's native plants and animals. This research reflects the data available at the time this evaluation was conducted. General Information Status: Submitted Screener: Emily Russell Evaluation Date: July 19, 2017 Plant Information Plant: Malus 'Donald Wyman' If the plant is a cultivar, how does its behavior differs from its parent's? 'Donald Wyman' has a broad, rounded habit. It is a larger crabapple that is sometimes used as a street tree. The flowers are white. The bright red fruit is noted to be "among the most persistent of all crabapples." It is also noted to be resistant to scab, cedar-apple rust, and mildew. 'Donald Wyman' was discovered as a chance seedling at the Arnold Arboretum around 1950. The exact parentage is unknown, but it is likely to have both Malus floribunda and Malus baccata in its genetic make-up. 'Donald Wyman' may be grafted onto a seedling or clonal rootstock. -
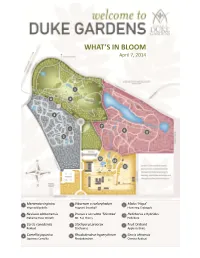
What's in Bloom
WHAT’S IN BLOOM April 7, 2014 5 4 6 2 7 1 9 8 3 12 10 11 1 Mertensia virginica 5 Viburnum x carlcephalum 9 Malus ‘Hopa’ Virginia Bluebells Fragrant Snowball Flowering Crabapple 2 Neviusia alabamensis 6 Prunus x serrulata ‘Shirotae’ 10 Helleborus x hybridus Alabama Snow Wreath Mt. Fuji Cherry Hellebore 3 Cercis canadensis 7 Stachyurus praecox 11 Fruit Orchard Redbud Stachyurus Apple cultivars 4 Camellia japonica 8 Rhododendron hyperythrum 12 Cercis chinensis Japanese Camellia Rhododendron Chinese Redbud WHAT’S IN BLOOM April 7, 2014 BLOMQUIST GARDEN OF NATIVE PLANTS Amelanchier arborea Common Serviceberry Sanguinaria canadensis Bloodroot Cornus florida Flowering Dogwood Stylophorum diphyllum Celandine Poppy Thalictrum thalictroides Rue Anemone Fothergilla major Fothergilla Trillium decipiens Chattahoochee River Trillium Hepatica nobilis Hepatica Trillium grandiflorum White Trillium Hexastylis virginica Wild Ginger Hexastylis minor Wild Ginger Trillium pusillum Dwarf Wakerobin Illicium floridanum Florida Anise Tree Trillium stamineum Blue Ridge Wakerobin Malus coronaria Sweet Crabapple Uvularia sessilifolia Sessileleaf Bellwort Mertensia virginica Virginia Bluebells Pachysandra procumbens Allegheny spurge Prunus americana American Plum DORIS DUKE CENTER GARDENS Camellia japonica Japanese Camellia Pulmonaria ‘Diana Clare’ Lungwort Cercis canadensis Redbud Prunus persica Flowering Peach Puschkinia scilloides Striped Squill Cercis chinensis Redbud Sanguinaria canadensis Bloodroot Clematis armandii Evergreen Clematis Spiraea prunifolia Bridalwreath -

Handling of Apple Transport Techniques and Efficiency Vibration, Damage and Bruising Texture, Firmness and Quality
Centre of Excellence AGROPHYSICS for Applied Physics in Sustainable Agriculture Handling of Apple transport techniques and efficiency vibration, damage and bruising texture, firmness and quality Bohdan Dobrzañski, jr. Jacek Rabcewicz Rafa³ Rybczyñski B. Dobrzañski Institute of Agrophysics Polish Academy of Sciences Centre of Excellence AGROPHYSICS for Applied Physics in Sustainable Agriculture Handling of Apple transport techniques and efficiency vibration, damage and bruising texture, firmness and quality Bohdan Dobrzañski, jr. Jacek Rabcewicz Rafa³ Rybczyñski B. Dobrzañski Institute of Agrophysics Polish Academy of Sciences PUBLISHED BY: B. DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES ACTIVITIES OF WP9 IN THE CENTRE OF EXCELLENCE AGROPHYSICS CONTRACT NO: QLAM-2001-00428 CENTRE OF EXCELLENCE FOR APPLIED PHYSICS IN SUSTAINABLE AGRICULTURE WITH THE th ACRONYM AGROPHYSICS IS FOUNDED UNDER 5 EU FRAMEWORK FOR RESEARCH, TECHNOLOGICAL DEVELOPMENT AND DEMONSTRATION ACTIVITIES GENERAL SUPERVISOR OF THE CENTRE: PROF. DR. RYSZARD T. WALCZAK, MEMBER OF POLISH ACADEMY OF SCIENCES PROJECT COORDINATOR: DR. ENG. ANDRZEJ STĘPNIEWSKI WP9: PHYSICAL METHODS OF EVALUATION OF FRUIT AND VEGETABLE QUALITY LEADER OF WP9: PROF. DR. ENG. BOHDAN DOBRZAŃSKI, JR. REVIEWED BY PROF. DR. ENG. JÓZEF KOWALCZUK TRANSLATED (EXCEPT CHAPTERS: 1, 2, 6-9) BY M.SC. TOMASZ BYLICA THE RESULTS OF STUDY PRESENTED IN THE MONOGRAPH ARE SUPPORTED BY: THE STATE COMMITTEE FOR SCIENTIFIC RESEARCH UNDER GRANT NO. 5 P06F 012 19 AND ORDERED PROJECT NO. PBZ-51-02 RESEARCH INSTITUTE OF POMOLOGY AND FLORICULTURE B. DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES ©Copyright by BOHDAN DOBRZAŃSKI INSTITUTE OF AGROPHYSICS OF POLISH ACADEMY OF SCIENCES LUBLIN 2006 ISBN 83-89969-55-6 ST 1 EDITION - ISBN 83-89969-55-6 (IN ENGLISH) 180 COPIES, PRINTED SHEETS (16.8) PRINTED ON ACID-FREE PAPER IN POLAND BY: ALF-GRAF, UL. -

Your List for Epcot's Food & Wine Festival Here
Africa Hops and Barley • Spicy Kenyan Vegan • New England Lobster Roll: Warm Lobster with Fresh Herb Corn and White Beans with Pigeon Peas, Quinoa & Ancient Grains Medley and Kachumbari Slaw (Plant-based) Mayonnaise and Griddled Roll • Piri Piri Skewered Shrimp with Citrus-scented Couscous • Southern Seafood Boil: Shrimp, Mussels, Crawfish, Potatoes, • Tusker Lager Corn, and Andouille Sausage • Jam Jar Sweet Shiraz • Freshly Baked Carrot Cake and Cream Cheese Icing • North Coast Bewing Co. Blue Star Wheat Appleseed Orchard • 3 Daughters Brewing A Wake Coffee Blonde Ale • Heavy Seas AmeriCannon APA • Charcuterie and Cheese Plate featuring Burton’s Maplewood Farm Applejack Brandy Barrel Aged Maple Syrup • Angry Orchard Rosé Hard Cider • Caramel-Apple Popcorn • Mer Soleil Reserve Chardonnay • Frozen Apple Pie (non-alcoholic) • Broadside Cabernet Sauvignon • Short’s Brewing Co. Mule Beer • Heavy Seas Brewery TropiCannon Citrus IPA • Parish Brewing Co. Ghost in the Machine Double IPA Islands of the Caribbean • 3 Daughters Raspberry Lemonade Dry Hard Cider • Ropa Vieja Empanada with Tomato Aïoli • Short’s Brewing Company Starcut Ciders Mosa Hard Cider • Jerk-spiced Chicken with Roasted Sweet Plantain Salad and Mango Chutney Yogurt • Ciderboys Banana Bliss Hard Cider • Flancocho: Passion Fruit Cake with Coconut Flan • Apple Blossom Sky: Fanta® Apple, Ginger Beer, Floodwall Apple Brandy, Burton’s Maplewood Farm Applejack • Red Stripe Lager Brandy Barrel Aged Syrup and Mini Marshmallows • Opici Family White Sangria • Frozen Mojito Brazil • Crispy -

American As Apple Pie: Cultural Significance of American Baked Goods
La Salle University La Salle University Digital Commons HON499 projects Honors Program Spring 2021 American as Apple Pie: Cultural Significance of American Baked Goods Madeline Jupina La Salle University, [email protected] Follow this and additional works at: https://digitalcommons.lasalle.edu/honors_projects Part of the Food Studies Commons, and the Sociology of Culture Commons Recommended Citation Jupina, Madeline, "American as Apple Pie: Cultural Significance of American Baked Goods" (2021). HON499 projects. 39. https://digitalcommons.lasalle.edu/honors_projects/39 This Honors Project is brought to you for free and open access by the Honors Program at La Salle University Digital Commons. It has been accepted for inclusion in HON499 projects by an authorized administrator of La Salle University Digital Commons. For more information, please contact [email protected]. American as Apple Pie: Cultural Significance of American Baked Goods Madeline Jupina La Salle University Spring 2021 1 Introduction What does the phrase “American as apple pie” mean, really? How did apple pie become synonymous with Americana, the same way bagels became a symbol of the New York Jewish deli and funnel cakes associated with hot summer nights at the state fair? The culture behind food, especially baked goods, is often overlooked—but really, food is culture. Food tastes evolve over time, a product of the same slow and constant changes that evolve a culture: changes in values, new arrivals, status markers, and abundance or scarcity. These same cultural components shape what comes and goes, what stays, and what becomes so iconic that it becomes an American symbol (Visser, 1999). I have chosen four baked goods in particular: apple pie; Yum- Yum Cake, also known as Depression Cake; beignets; and bagels for their various intersections in American culture. -

The Masonic Homes Orchard
THE MASONIC VILLAGE FARM MARKET 310 Eden View Road, ELIZABETHTOWN PA 17022 PHONE (717) 361-4520 MVFarmmarket.com 2020 PICK-YOUR-OWN SCHEDULE Follow us on Facebook for up to date harvest information! Pick-Your-Own: Thursday through Saturday from 9:00 A.M. to 3:00 P.M. & Sunday 12-3 during the dates listed. Pumpkins are only available on specified weekends. Due to health regulations, pets are not allowed in the orchard or the Farm Market. • Thursday & Friday: Please stop in the Farm Market to receive instructions on picking locations and to get your picking supplies. Please bring your apples into the Farm Market to check out before you leave. • Saturday & Sunday: You may go directly to the orchard for picking supplies and checkout • PYO Apple: 99¢ lb., PYO Pumpkins 65¢ lb., PYO Peach: $1.25 lb., Sunflowers $1 stem Variety and quantity availability is on a first come basis. Mother Nature decides the volume on the trees, what date the fruit ripens and when it falls off the trees. We’re just along for the ride. Peach harvest dates are very difficult to predict due to temperature fluctuations. Please call the Farm Market for exact varieties available. July 30-Aug. 2……Pick Your Own Red Haven Peaches, Sunflowers…watch Facebook for an exact start date August 6-9……………..….Red Haven Peaches, Sunflowers August 20-23..………….…Red Haven Peaches, Possibly Sun High Peaches, Sunflowers August 27-30……………...Sun High Peaches, Gala and Jonamac Apples (Possibly Honeycrisp) September 3-6…………….Cresthaven Peaches, Honeycrisp, Gala, Cortland and Jonamac apples September -

Native Trees of Georgia.Pdf
NATIVE TREES OF GEORGIA By G. Norman Bishop Professor of Forestry George Foster Peabody School of Forestry University of Georgia Currently Named Daniel B. Warnell School of Forest Resources University of Georgia GEORGIA FORESTRY COMMISSION J. Frederick Allen Director Tenth Printing - 2000 Revised Edition 2 FOREWARD This manual has been prepared in an effort to give to those interested in the trees of Georgia a means by which they may gain a more intimate knowledge of the tree species. Of about 250 species native to the state, only 92 are described here. These were chosen for their commercial importance, distribution over the state or because of some unusual characteristic. Since the manual is intended primarily for the use of the layman, technical terms have been omitted wherever possible; however, the scientific names of the trees and the families to which they belong, have been included. It might be explained that the species are grouped by families, the name of each occurring at the top of the page over the name of the first member of that family. Also, there is included in the text, a subdivision entitled KEY CHARACTERISTICS, the purpose of which is to give the reader, all in one group, the most outstanding features whereby he may more easily recognize the tree. ACKNOWLEDGEMENTS The author wishes to express his appreciation to the Houghton Mifflin Company, publishers of Sargent’s Manual of the Trees of North America, for permission to use the cuts of all trees appearing in this manual; to B. R. Stogsdill for assistance in arranging the material; to W. -

Guidance Document for Tree Conservation, Landscape, and Buffer Requirements Article III Section 3.2
Guidance Document For Tree Conservation, Landscape, and Buffer Requirements Article III Section 3.2 Table of Contents Table of Contents Revision History ............................................................................................................................................ 1 Technical Standards ...................................................................................................................................... 2 1. Tree Measurements .......................................................................................................................... 2 2. Specimen Trees ................................................................................................................................. 4 3. Boundary Tree ................................................................................................................................... 4 4. Tree Density Calculation ................................................................................................................... 5 Tree Removal Requirements ...................................................................................................................... 11 Tree Care ..................................................................................................................................................... 12 1. Planting ........................................................................................................................................... 12 2. Mulching ........................................................................................................................................