Maned Wolf Husbandry Manual 2007 Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2018 Infant Mortality Annual Report 2 Ohio Infant Mortality Report 2018 TABLE of CONTENTS
2018 Infant Mortality Annual Report 2 Ohio Infant Mortality Report 2018 TABLE OF CONTENTS EXECUTIVE SUMMARY 4 SECTION 1: GENERAL FINDINGS 5-10 Ohio Infant Mortality Rate by Race and Ethnicity (2016-2018) 5 Trends in Ohio Infant Mortality (2009-2018) 6 Ohio Five-Year Average Infant Mortality Rates by County 8 Neonatal and Postneonatal Mortality Rates 9-10 SECTION 2: A DEEPER LOOK 11-19 Infant Mortality by Duration of Gestation and Birthweight 11-12 Risk Factors among Ohio Infant Deaths 13-14 Leading Causes of Infant Deaths 15-16 Prematurity-Related Infant Deaths 17 External Injury-Related Infant Deaths 18 Deaths to Infants Less Than 24 Weeks Gestation 19 DATA SOURCES AND METHODS 20 DEFINITIONS 21-22 REFERENCES 23 APPENDICES 24-45 Appendix A: The Ohio Equity Institute (OEI) 24-25 Appendix B: Sleep-Related Deaths 26-29 Appendix C: Supplementary Data Tables 30-45 Section 1: County-Level Infant Mortality Data 30-36 Section 2: A Deeper Look 37-45 3 EXECUTIVE SUMMARY Ohio has identified infant mortality as a priority in its 2017-2019 State Health Improvement Plan (SHIP)1. Infant mortality is the death of an infant before his or her first birthday. The Infant Mortality Rate is the number of infant deaths per 1,000 live births. The Infant Mortality Rate not only serves as a key indicator of maternal and infant health but is also an important measure of the health status of a community. In 2018, the infant mortality rate fell to 6.9 from 7.2 in 2017 for all races. -

Magazine Fall 2018
Fall 2018 IN THIS ISSUE: Our Mission: Double Cross Foster To preserve and protect Mexican wolves, Pages 6, 7, 8 red wolves and other wild canid species, Red Wolf Update Page 9 with purpose and passion, through Citizen Conservation in Action carefully managed breeding, reintroduction Page 10 and inspiring education programs. The Week of the Wolf Presented by Emerson Page 11 EXECUTIVE DIRECTOR’S MESSAGE 2018 Events Nov. 17 Dear Friends of the Endangered Wolf Center, Members’ Day I’ve spent the summer enjoying what the Nov. 24 Endangered Wolf Center has to offer to our guests Holiday Boutique and community. Hearing the laughter of our camp kids while walking the grounds, seeing the tour guests “oh and ah” over our resident animals and, more personally, hearing the wonder and 2019 Events excitement in my daughter’s voice during the car Feb. 15 ride home from her first wolf camp experience. Trivia Night Through our Center and thanks to your support, March 31 so many lives are being touched – and endangered Volunteer Appreciation Dinner wildlife is being saved. TBD Imagine if you could help save an entire species. Polo Classic I’d like to invite you to be a Wildlife Hero. Through your support, you can Oct. 12 help us save not one, but two endangered species as we increase our focus on Wolf Fest our history-making rewilding efforts. Nov. 16 • Rewilding Efforts: As you can imagine, rewilding is not without great Members’ Day challenges. Successful reintroductions are accomplished through a combination of ongoing breeding of genetically valuable wolves; the Nov. -

Low Birthweight
OECD Health Statistics 2021 Definitions, Sources and Methods Low birthweight Number of live births weighing less than 2500 grams as a percentage of the total number of live births. Sources and Methods Australia Source: Australian Institute of Health and Welfare. From 1991: Australia’s mothers and babies reports available to download at https://www.aihw.gov.au/reports- statistics/population-groups/mothers-babies/reports. - From 2013 onwards, the Australian Institute of Health and Welfare has produced the data. - From 1991 to 2012, the National Perinatal Epidemiology and Statistics Unit produced the data on behalf of the Australian Institute of Health and Welfare. Before 1991: State and Territory maternal and perinatal collections. Break in time series in 1991: Before 1991, data refer to selected states and territories only and have total births (live births + stillbirths) as a denominator. Further information: https://www.aihw.gov.au/reports-statistics/population-groups/mothers-babies/overview. Austria Source: Statistics Austria, Gesundheitsstatistisches Jahrbuch (Health Statistics Report), Lebendgeborene nach Geburtsgewicht (Live births by birth weight) (several issues). Further information: http://www.statistik.at/web_en/. Belgium Source: Federal Public Service Economic Affairs - Directorate General for statistical and economic information (former National Statistical Institute). Methodology: Since 2010, the official numbers for livebirths and deaths are coming from the Population National Register (and not exclusively from vital registration). Livebirths and deaths of residents taking place in foreign countries are therefore included in the statistics. Canada Source: Statistics Canada. Canadian Vital Statistics Birth Database. From 1979: Table 13-10-0404-01 (formerly CANSIM 102-4005). 1961-1978: Births, 1991, Cat. No. -

Verzeichnis Der Europäischen Zoos Arten-, Natur- Und Tierschutzorganisationen
uantum Q Verzeichnis 2021 Verzeichnis der europäischen Zoos Arten-, Natur- und Tierschutzorganisationen Directory of European zoos and conservation orientated organisations ISBN: 978-3-86523-283-0 in Zusammenarbeit mit: Verband der Zoologischen Gärten e.V. Deutsche Tierpark-Gesellschaft e.V. Deutscher Wildgehege-Verband e.V. zooschweiz zoosuisse Schüling Verlag Falkenhorst 2 – 48155 Münster – Germany [email protected] www.tiergarten.com/quantum 1 DAN-INJECT Smith GmbH Special Vet. Instruments · Spezial Vet. Geräte Celler Str. 2 · 29664 Walsrode Telefon: 05161 4813192 Telefax: 05161 74574 E-Mail: [email protected] Website: www.daninject-smith.de Verkauf, Beratung und Service für Ferninjektionsgeräte und Zubehör & I N T E R Z O O Service + Logistik GmbH Tranquilizing Equipment Zootiertransporte (Straße, Luft und See), KistenbauBeratung, entsprechend Verkauf undden Service internationalen für Ferninjektionsgeräte und Zubehör Vorschriften, Unterstützung bei der Beschaffung der erforderlichenZootiertransporte Dokumente, (Straße, Vermittlung Luft und von See), Tieren Kistenbau entsprechend den internationalen Vorschriften, Unterstützung bei der Beschaffung der Celler Str.erforderlichen 2, 29664 Walsrode Dokumente, Vermittlung von Tieren Tel.: 05161 – 4813192 Fax: 05161 74574 E-Mail: [email protected] Str. 2, 29664 Walsrode www.interzoo.deTel.: 05161 – 4813192 Fax: 05161 – 74574 2 e-mail: [email protected] & [email protected] http://www.interzoo.de http://www.daninject-smith.de Vorwort Früheren Auflagen des Quantum Verzeichnis lag eine CD-Rom mit der Druckdatei im PDF-Format bei, welche sich großer Beliebtheit erfreute. Nicht zuletzt aus ökologischen Gründen verzichten wir zukünftig auf eine CD-Rom. Stattdessen kann das Quantum Verzeichnis in digitaler Form über unseren Webshop (www.buchkurier.de) kostenlos heruntergeladen werden. Die Datei darf gerne kopiert und weitergegeben werden. -

Report Highlights
Consensus Study Report October 2020 HIGHLIGHTS A Research Strategy to Examine the Taxonomy of the RED WOLF Dramatic reductions in the population of modern WHY DO MORE RESEARCH ON red wolf, Canis rufus in the eastern United States led to THE RED WOLF? their re-introduction by the U.S. Fish and Wildlife Service There are three possible explanations for the significant (USFWS) into North Carolina in the early 1980s. Since amount of coyote ancestry in modern red wolves. First, then, USFWS has managed a population of red wolves that the extant red wolves may be hybrids between coyotes was established from re-introduced individuals. However, and either gray wolves or what were once red wolves. subsequent genetic studies of the managed population Hybridization may have occurred long ago or in more indicated that these wolves displayed evidence of significant recent years. Evidence suggests that some hybridization coyote ancestry. This discovery raised the issue of whether certainly occurred in the 20th century as red wolf the extant managed1 population in North Carolina was a populations dwindled in size while coyotes expanded valid species that was eligible for conservation and recovery. their range eastward. Second, the extant red wolves may In March 2019, at the request of USFWS, the National have diverged from coyotes recently enough that they Academies of Sciences, Engineering, and Medicine issued continue to share alleles, even though each species has its a report that provided evidence that retained the existing own forward evolutionary trajectory— a situation referred taxonomic designation of the red wolf and reinforced the to as incomplete lineage sorting. -

EPIDEMIOLOGY of SELECTED INFECTIOUS DISEASES in ZOO-UNGULATES: SINGLE SPECIES VERSUS MIXED SPECIES EXHIBITS Carolina Probst
EPIDEMIOLOGY OF SELECTED INFECTIOUS DISEASES IN ZOO-UNGULATES: SINGLE SPECIES VERSUS MIXED SPECIES EXHIBITS Carolina Probst, DVM,* Heribert Hofer, MSc, PhD, Stephanie Speck, DVM, PhD, and Kai Frölich, DVM, PhD1 Institute for Zoo and Wildlife Research, Alfred-Kowalke Strasse 17, 10315 Berlin, Germany Reprinted with permission. American Association of Zoo Veterinarians, 2005. Joint Annual Conference. Abstract The study analyses the epidemiology of selected infectious diseases of 65 different species within the four families of bovids, cervids, camelids and equids in one czech and nine German zoos. It is based on a survey of all epidemiologic data since 1998. Furthermore 900 blood samples taken between 1998 and 2005 are screened for the presence of antibodies against selected viral and bacterial pathogens. The results are linked to the epidemiologic data. Introduction The concept of mixed species exhibits increasingly becomes important in European zoos. It is an important form of behavioral enrichment, it optimizes the use of space and it is of great educational value for visitors, giving them an impression of ecological connections. But until now it has not been elucidated whether the kind of exhibit may lead to an increase in the prevalence of specific infections. The aims of this study are to evaluate the exposure of zoo-ungulates to a variety of disease pathogens that can be transmitted between different species and to assess the epidemiology of mixed exhibits. We are interested in the following questions: 1. Which selected infectious agents are zoo ungulates exposed to? 2. What is the seroprevalence against these agents? 3. Is there a correlation between seroprevalence and the following factors: - animal exhibition system (single species / mixed species exhibit) - population density and animal movements - interspecific contact rates 4. -

Reciprocal Zoos and Aquariums
Reciprocity Please Note: Due to COVID-19, organizations on this list may have put their reciprocity program on hold as advance reservations are now required for many parks. We strongly recommend that you call the zoo or aquarium you are visiting in advance of your visit. Thank you for your patience and understanding during these unprecedented times. Wilds Members: Members of The Wilds receive DISCOUNTED or FREE admission to the AZA-accredited zoos and aquariums on the list below. Wilds members must present their current membership card along with a photo ID for each adult listed on the membership to receive their discount. Each zoo maintains its own discount policies, and The Wilds strongly recommends calling ahead before visiting a reciprocal zoo. Each zoo reserves the right to limit the amount of discounts, and may not offer discounted tickets for your entire family size. *This list is subject to change at any time. Visiting The Wilds from Other Zoos: The Wilds is proud to offer a 50% discount on the Open-Air Safari tour to members of the AZA-accredited zoos and aquariums on the list below. The reciprocal discount does not include parking. If you do not have a valid membership card, please contact your zoo’s membership office for a replacement. This offer cannot be combined with any other offers or discounts, and is subject to change at any time. Park capacity is limited. Due to COVID-19 advance reservations are now required. You may make a reservation by calling (740) 638-5030. You must present your valid membership card along with your photo ID when you check in for your tour. -

Red Wolves: Creating Economic Opportunity Through Ecotourism in Rural North Carolina
Red Wolves: Creating Economic Opportunity Through Ecotourism in Rural North Carolina Report By Dr. Gail Y. B. Lash & Pamela Black Ursa International For Defenders of Wildlife Washington, DC February 2005 Red Wolves: Creating Economic Opportunity Through Ecotourism in Rural North Carolina Report By Dr. Gail Y. B. Lash & Pamela Black Ursa International Published By Defenders of Wildlife Washington, DC February 2005 Defenders of Wildlife 1130 Seventeenth Street NW Washington, DC 20036-4604 USA phone: 1-202-682-9400 web: http://www.defenders.org Ursa International 366 Oakland Ave., SE Atlanta, GA 30312-2233 USA phone: 1-404-222-9595 web: http://www.ursainternational.org Red Wolf Ecotourism Report, p. 1 TABLE OF CONTENTS Page Foreword .............................................................................................................................4 Executive Summary.............................................................................................................5 List of Tables .......................................................................................................................7 List of Figures......................................................................................................................8 List of Abbreviations ...........................................................................................................9 Introduction........................................................................................................................10 Purpose of Study....................................................................................................10 -

The Red and Gray Fox
The Red and Gray Fox There are five species of foxes found in North America but only two, the red (Vulpes vulpes), And the gray (Urocyon cinereoargentus) live in towns or cities. Fox are canids and close relatives of coyotes, wolves and domestic dogs. Foxes are not large animals, The red fox is the larger of the two typically weighing 7 to 5 pounds, and reaching as much as 3 feet in length (not including the tail, which can be as long as 1 to 1 and a half feet in length). Gray foxes rarely exceed 11 or 12 pounds and are often much smaller. Coloration among fox greatly varies, and it is not always a sure bet that a red colored fox is indeed a “red fox” and a gray colored fox is indeed a “gray fox. The one sure way to tell them apart is the white tip of a red fox’s tail. Gray Fox (Urocyon cinereoargentus) Red Fox (Vulpes vulpes) Regardless of which fox both prefer diverse habitats, including fields, woods, shrubby cover, farmland or other. Both species readily adapt to urban and suburban areas. Foxes are primarily nocturnal in urban areas but this is more an accommodation in avoiding other wildlife and humans. Just because you may see it during the day doesn’t necessarily mean it’s sick. Sometimes red fox will exhibit a brazenness that is so overt as to be disarming. A homeowner hanging laundry may watch a fox walk through the yard, going about its business, seemingly oblivious to the human nearby. -

Audubon Nature Institute 2016
CONSERVATION Celebrating Audubon Nature Institute Each day, our partners here at the Wonders home and around the globe of Nature work with us on fulfilling our 2016 shared goals. All eight objectives of the Audubon Nature Institute mission have conservation at their core, particularly our pledges to preserve native Louisiana habitats and to enhance the care and survival of wildlife through research and conservation. That’s why we wanted to show you the scope of Audubon’s conservation commitment through this report. These projects are top of mind for us every day, and we work on them together—donors, members, guests, employees, and peer organizations around the world. From the smallest act of recycling a piece of paper to multi-national coalitions saving species oceans away, we know we must keep pushing forward. The stakes are high, and together, we are making progress. Sincerely, Ron Forman President and CEO Audubon Nature Institute FOUNDING SUPPORTER 2016 NEWS of AZA’s SAFE Program Audubon is New Elephant Environment As the world’s largest land mammals, elephants have an active a profound effect on our ecosystem, so Audubon is $919,908 participant in the Wildlife part of a nationwide initiative of zoos banding together Dedicated to conservation initiatives Conservation to fund elephant conservation. At Audubon Zoo our Society’s elephants settled in recently to a spacious new habitat monumental that raises awareness to our 850,000 annual visitors 96 Elephants and shows people how they can help keep these initiative. animals from disappearing -

2021 Fur Harvester Digest 3 SEASON DATES and BAG LIMITS
2021 Michigan Fur Harvester Digest RAP (Report All Poaching): Call or Text (800) 292-7800 Michigan.gov/Trapping Table of Contents Furbearer Management ...................................................................3 Season Dates and Bag Limits ..........................................................4 License Types and Fees ....................................................................6 License Types and Fees by Age .......................................................6 Purchasing a License .......................................................................6 Apprentice & Youth Hunting .............................................................9 Fur Harvester License .....................................................................10 Kill Tags, Registration, and Incidental Catch .................................11 When and Where to Hunt/Trap ...................................................... 14 Hunting Hours and Zone Boundaries .............................................14 Hunting and Trapping on Public Land ............................................18 Safety Zones, Right-of-Ways, Waterways .......................................20 Hunting and Trapping on Private Land ...........................................20 Equipment and Fur Harvester Rules ............................................. 21 Use of Bait When Hunting and Trapping ........................................21 Hunting with Dogs ...........................................................................21 Equipment Regulations ...................................................................22 -
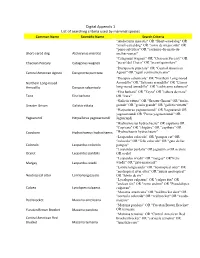
Digital Appendix 1 List of Searching Criteria Used by Mammal Species
Digital Appendix 1 List of searching criteria used by mammal species Common Name Scientific Name Search Criteria "Atelocynus microtis" OR "Short-eared dog" OR "small-eared dog" OR "zorro de oreja corta" OR "perro selvático" OR "cachorro-do-mato-de- Short-eared dog Atelocynus microtis orelhas-curtas" "Catagonus wagneri" OR "Chacoan Peccary" OR Chacoan Peccary Catagonus wagneri "pecarí del Chaco" OR "pecarí quimilero" “Dasyprocta punctate” OR "Central American Central American Agouti Dasyprocta punctata Agouti" OR "agutí centroamericano" “Dasypus sabanicola” OR "Northern Long-nosed Northern Long-nosed Armadillo" OR "Savanna armadillo" OR "Llanos Armadillo Dasypus sabanicola long-nosed armadillo" OR "cachicamo sabanero" “Eira barbara” OR "Tayra" OR "cabeza de mate" Taira Eira barbara OR "irara" “Galictis vittata” OR "Greater Grison" OR "furão- Greater Grison Galictis vittata grande" OR "grisón grande" OR "galictis vittatta" “Herpailurus yagouaroundi” OR Yaguarundi OR yagouaroundi OR "Puma yagouaroundi" OR Yaguarundi Herpailurus yagouaroundi Jaguarundi "Hydrochoerus hydrochaeris" OR capybara OR "Capivara" OR "chigüire" OR "capibara" OR Capybara Hydrochoerus hydrochaeris "Hydrochaeris hydrochaeris" “Leopardus colocolo” OR "pampas cat" OR "colocolo" OR "felis colocolo" OR "gato de las Colocolo Leopardus colocolo pampas" "Leopardus pardalis" OR jaguatirica OR ocelote Ocelot Leopardus pardalis OR ocelot “Leopardus wiedii” OR "margay" OR"Felis Margay Leopardus wiedii wiedii" OR "gato-maracajá" “Lontra longicaudis” OR "neotropical otter" OR "neotropical