Nutritional Adequacy and Performance of Raw Food Diets in Kittens
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Breeding Policy
PERSIAN LONGHAIR BREED ADVISORY COMMITTEE Breeding Policy SUPREME UK OG & IMP GR CH GEMKIN STARWIND OVERALL SUPREME EXHIBIT 2012 & 2013 CONTENTS Introduction Origins of the Breed Pattern Groups Genetic Make-up Breeding System Inbreeding Genetic Defects Grooming Introduction With the formation of a consolidated BAC for Persian Longhairs, the requirement to pro- duce a breeding policy has given the BAC the opportunity to review the Registration Poli- cies and Standards of Points for Persians. Some of the policies for the individual pattern groups have not been reviewed or revised for some years. During this time, the Fancy has altered considerably, with the number of Persians being shown dropping dramati- cally, with a consequent reduction in breeders and breeding cats but also, on the plus side, the ability to show longhairs from Exotic or Exotic/Persian breeding at championship level in the section. The aim of this breeding policy is to give advice and guidance to breeders to enable them to observe what is considered “best practice” in breeding Persian Longhairs. The aims of these amendments are to: open up the gene-pool enable breeders to outcross make it easier for breeders to import outcross bloodlines The over-riding factor should always be to maintain health, and preserve the unique qualities of this stunning breed, coat colour, length and texture, beautiful large, round eyes and sweet facial expression, which makes them sought after both for showing and as wonderful family pets. Origins of the Breed The breed’s name refers to Persia, the former name of Iran, where similar cats are found. -

TICACATS German American Cat Club E.V
TICACATS German American Cat Club e.V. Proudly Presents Carnival of Cats 2020 22 - 23 February 2020 In Holzminden, Germany Stadthalle Holzminden Richter/Judges Samstag Sonntag Saturday Sunday Steven Savant USA AB AB Party Cats Luiz Paulo Facciolo BR AB AB RD, LP, RB Francine Hicks USA AB AB Female Congress Sue Hart-Jones UK AB AB Carnival Cats Nicki Fenwick-Raven UK SP SP Ragcoons Andreas Kretschmer-Kraiczek D AB AB Male Congress Master Clerk Ring Clerks Magdalena Marié PL Arie Groenewegen NL Monika Kolodziej PL Ring Clerks Jakub Kruszona-Zawadzski PL Desiree Alderliesten NL Johann Reuser NL George Cherrie NL Show Manager Ralph Stadter Assistant Show Manager Amy Stadter Entry Clerk Sabine Hübner Ausstellungskommittee Sabine Hübner und Peter Bitomsky Show Committee André Lieske Monja Bülow und Andreas Gellert Nicole Quaiser Petra und Norbert Garbe Amy und Ralph Stadter Tierarzt Dr. Krieger, Holzminden LH KITTEN SATURDAY SUNDAY SS LPF FH SHJ AKR NFR SS LPF FH SHJ AKR NFR BRITISH LONGHAIR TRADITIONAL TORTIE & WHITE DIVISION CHOCOLATE TORTIE&WHITE 0A ENCHANTEDPAWS MARGOT ROBBIE 0.6 F 0A SBV 072719 015 BORN: JUL 27, 2019 CH TER HEIDE’S ZINO SBT 091217 043 CH XIJA ELANDREA SBV 033118 063 1 1 1 1 1 1 B/O: ELLEN GREENAWAY 1 1 1 1 1 1 EW EW 0A 0A 0A 0A 0A 0A BEST OF DIVISION / BREED 0A 0A 0A 0A 0A 0A LAPERM TRADITIONAL TORTIE & WHITE DIVISION LILAC TORTIE&WHITE 1 Smeraldas Viva la Vida 0.7 F 1 PENDING BORN: JUL 01, 2019 FRISSON BC KAVIR SMERALDAS BC PERLE 1 1 1 1 1 1 B: SYLVIE GROENVELD 1 1 1 1 1 1 EN O: SYLVIA GROENVELD EN 1 1 1 1 1 1 BEST OF -

NOVEMBER 1968 - Sof FANCY the E"Otic II •
NOVEMBER 1968 - SOf FANCY The E"otic II •... 10 Few Lines 12 C!S Reporter . .. 5 New Research 13 Cots Bookshelf 14 Tl Fonciers Forum 24 Birth Control 18 Picture of Month .. 17 Show Colendor 26 Glomour Puss 22 Whot's Mew. .•... 16 Show Reports 29 FEATURES Dr. LesCallette... 20 F ARTICLES Editorial. • . • •• 4 Arobello .••••.. 31 The Cot Artist . .. 8 Readers Write ••. 6 Bock Fence •..•• 38 I 100 SUGGESTIONS FOR CAT CHRISTMAS GIFTS L=:==::::=!!!!!::::.----::=JI 10 NOVEMBER 1968 THE MOD PERSIAN IN THE MINI~SKIRT".III CPA'S ANSWER admission of illicit breeding, nor what could be described as a Persian Perceiving this dilemma, one should anything of the sort be read Shorthair. The sine qua non is sufficient national association, The Cat Fanciers' into this action. It means one thing Persian somewhere in the background, Association, Inc., took a radical and and one thing only. The cat in questions not necessarily in the immediate enlightened step. It moved to protect has Persian characteristics, probably parentage, to give the Persian type the Domestic-known to it as the because of Persian ancestry in the and head. Since long hair is recessive American Shorthair-by bringing its "unknown parentage" portion of the to short hair in any case, retaining the standard back into line with its true pedigree, and can more properly be short coat while enhancing the Persian characteristics. judged in the Exotic Shorthair class conformation is not too difficult. The At the same time, by recognizing whose standard it meets than in the most obvious choice of Shorthair to for championship competition a breed American Shorthair class whose stand use is the American. -

Feline Lower Urinary Tract Disease from Wikipedia, the Free Encyclopedia
Log in / create account article discussion edit this page history Feline lower urinary tract disease From Wikipedia, the free encyclopedia Feline lower urinary tract disease (FLUTD) is a term that is used to cover many problems of the feline urinary tract, including stones and cystitis. The term feline urologic syndrome (FUS) is an older term which is still sometimes used for this condition. The condition can lead to plugged navigation penis syndrome also known as blocked cat syndrome. It is a common disease in adult cats, though it can strike in young cats too. It may Main page present as any of a variety of urinary tract problems, and can lead to a complete blockage of the urinary system, which if left untreated is fatal. Contents FLUTD is not a specific diagnosis in and of itself, rather, it represents an array of problems within one body system. Featured content Current events FLUTD affects cats of both sexes, but tends to be more dangerous in males because they are more susceptible to blockages due to their longer, Random article narrower urethrae. Urinary tract disorders have a high rate of recurrence, and some cats seem to be more susceptible to urinary problems than others. search Contents 1 Symptoms Go Search 2 Causes interaction 3 Treatment About Wikipedia 4 Further reading Community portal 5 External links Recent changes Contact Wikipedia Symptoms [edit] Donate to Wikipedia Help Symptoms of the disease include prolonged squatting and straining during attempts to urinate, frequent trips to the litterbox or a reluctance to leave toolbox the area, small amounts of urine voided in each attempt, blood in the urine, howling, crying, or other vocalizations. -
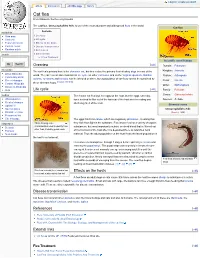
Cat Flea from Wikipedia, the Free Encyclopedia
Log in / create account article discussion edit this page history Cat flea From Wikipedia, the free encyclopedia The cat flea, Ctenocephalides felis, is one of the most abundant and widespread fleas in the world. Cat flea Contents navigation Main page 1 Overview Contents 2 Life cycle Featured content 3 Effects on the hosts Current events 4 Disease transmission Random article 5 References search 6 External links 6.1 Flea Treatment Scientific classification Go Search Overview [edit] Domain: Eukaryota interaction The cat flea's primary host is the domestic cat, but this is also the primary flea infesting dogs in most of the Kingdom: Animalia About Wikipedia world. The cat flea can also maintain its life cycle on other carnivores and on the Virginia opossum. Rabbits, Phylum: Arthropoda Community portal rodents, ruminants and humans can be infested or bitten, but a population of cat fleas cannot be sustained by Recent changes Class: Insecta [citation needed] Contact Wikipedia these aberrant hosts. Order: Siphonaptera Donate to Wikipedia Help Life cycle [edit] Family: Pulicidae Genus: Ctenocephalides toolbox The female cat flea lays her eggs on the host, but the eggs, once dry, What links here have evolved to filter out of the haircoat of the host into the resting and Species: C. felis Related changes sheltering area of the host. Upload file Binomial name Special pages Ctenocephalides felis Printable version (Bouché, 1835) Permanent link Cite this page The eggs hatch into larvae, which are negatively phototaxic, meaning that languages Photo showing some they hide from light in the substrate. Flea larvae feed on a variety of organic characteristics used to identify from Deutsch substances, but most importantly subsist on dried blood that is filtered out other fleas, including genal comb Français of the haircoat of the host after it is deposited there as adult flea fecal Nederlands material. -

National Breed Standards
NATIONAL BREED STANDARDS © ANCATS 2017 ANCATS National Breed Standards 2017 1 INDEX ........................................................................................................................................................................................................................................................................... 2 GLOSSARY ................................................................................................................................................................................................................................................................. 5 PREFACE .................................................................................................................................................................................................................................................................. 10 The Condition of the Cat .................................................................................................................................................................................................................. 11 Judging Disqualification Faults ........................................................................................................................................................................................................ 12 General faults in all breeds precluding a Challenge or Best in Show ............................................................................................................................................. -

Gross Causes of Neonatal Mortality in Devon Rex and Persian Kittens By
Gross Causes of Neonatal Mortality in Devon Rex and Persian Kittens by Holly Omoto A THESIS submitted to Oregon State University Honors College in partial fulfillment of the requirements for the degree of Honors Baccalaureate of Science in Animal Sciences (Honors Scholar) Presented May 16, 2019 Commencement June 2019 AN ABSTRACT OF THE THESIS OF Holly Omoto for the degree of Honors Baccalaureate of Science in Animal Sciences presented on May 16, 2019. Title: Gross Causes of Neonatal Mortality in Devon Rex and Persian Kittens. Abstract approved: _____________________________________________________ Michelle Kutzler Post-mortem examinations were performed on 29 deceased Persian kittens from 13 different litters, and 30 deceased Devon Rex kittens from 18 different litters in order to identify gross anatomical abnormalities. Fifteen kittens were stillborn (8 Devon Rex, 7 Persian), 17 kittens died during the first day of life (16 Devon Rex, 1 Persian), 14 kittens died later in the first week of life (5 Devon Rex, 9 Persian), and 13 kittens died during the second week of life (1 Devon Rex, 12 Persian). The average age at death excluding stillborn kittens was 5.2±4.7 days for both breeds, 1.8±2.0 days for Devon Rex kittens, and 8.5±4.2 days for Persian kittens. The most common sign reported by the cat breeders before death was dyspnea, with 12 kittens affected (11 Devon Rex, 1 Persian). Postmortem examination findings included pyothorax (9 Devon Rex kittens), pigmenturia (4 Devon Rex, 2 Persian kittens). It is estimated that at least 22.0% (13/59) of neonatal mortalities were due to infectious etiologies, at least 6.8% (4/59) were due to noninfectious etiologies, (n=7), and up to 71.2% (42/59) were classified as idiopathic. -

Isolation of Feline Herpesvirus-1 and Feline Calicivirus from Healthy Cats in Swedish Breeding Catteries
Journal of Feline Medicine and Surgery (2005) 7, 325e331 doi:10.1016/j.jfms.2005.03.002 Isolation of feline herpesvirus-1 and feline calicivirus from healthy cats in Swedish breeding catteries 1 2 Bodil Stro¨ m Holst DVM, PhD, Dipl ECAR *, Louise T Berndtsson DVM , 1 Lena Englund DVM, PhD 1Department of Companion animals Feline calicivirus (FCV) could be isolated from four cats (2.6%) and feline and horses, National Veterinary herpesvirus-1 (FHV) from none of 152 clinically healthy cats from 22 Swedish Institute, SE-751 89 Uppsala, breeding catteries. These cats had all previously shown signs of respiratory tract Sweden disease or conjunctivitis, although several years ago. The results suggest that 2Department of Virology, National carriers of FCV and FHV were uncommon in Swedish breeding catteries studied. Veterinary Institute, SE-751 89 Prevalence rates in other European countries and North America are usually Uppsala, Sweden higher, especially of FCV. The lower prevalence rates in our study might be explained by test group selection, differences in factors such as management, environment, or genetic constitution of the cats, or by sample handling. It was concluded that the presence of an FCV shedder in the cattery does not mean that all cats in the group are infected, but special measures are recommended to avoid infection of susceptible cats. Date accepted: 2 February 2005 Ó 2005 ESFM and AAFP. Published by Elsevier Ltd. All rights reserved. espite the widespread use of vaccines Clinical disease caused by the two viruses is against feline herpesvirus-1 (FHV, feline commonly most severe in young animals. -

Apparently There Is Great Pleasure in Maintaining an Aura of Mys- Tery Around the Beginnings of a Breed
THE EXOTIC SHORTHAIR Rosemonde S. Peltz and Joan S. Uzee THE BEGINNINGS: CAUSE AND EFFECT Apparently there is great pleasure in maintaining an aura of mys- tery around the beginnings of a breed. How ironic it is that a breed with the fanciful name of Exotic Shorthair was created to turn sin- ners into saints and to provide baptism for the ill-begotten. Perhaps in years hence the beginnings of this breed will have its own legend; but for now the record should be kept straight. The background of the Exotic Shorthair begins in England and concerns not only the British Shorthair there, but also the silver shorthairs in this country. It had been a common practice in England to cross Persian with domestic cats to pro- duce a cat of Persian type with a short, thick, dense coat. The cats in England were of many colors, but the primary cross that occurred in the United States involved silver and the silver tabby pat- itish Blue. Cream Pensylva tern. The color silver, which results from St3:tip of Heritage (Imp.) Br. the chinchilla alleles reducing yellow J. Richards, Ow. D. Hansen & melanin faster than black, occurred first R. Peitz. as a mutation in the Persian cat. There- fore, most, if not all, silver shorthairs have resulted from a transfer of silver from longhair to shorthair. It is conceivable, of course, that chinchilla occurred as a separate mutation later in shorthairs, but this would be extremely difficult either to prove or disapprove and is extremely unlikely. Year Book, 1972 403 The practice of crossing Persian to Domestic Shorthair was quite prevalent in the 1950's and continues to this day. -
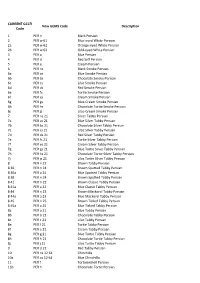
GEMS CODE LIST.Xlsx
CURRENT GCCFI New GEMS Code Description Code 1 PER n Black Persian 2 PER w 61 Blue-eyed White Persian 2a PER w 62 Orange-eyed White Persian 2b PER w 63 Odd-eyed White Persian 3 PER a Blue Persian 4 PER d Red Self Persian 5 PER e Cream Persian 6 PER ns Black Smoke Persian 6a PER as Blue Smoke Persian 6b PER bs Chocolate Smoke Persian 6c PER cs Lilac Smoke Persian 6d PER ds Red Smoke Persian 6e PER fs Tortie Smoke Persian 6f PER es Cream Smoke Persian 6g PER gs Blue-Cream Smoke Persian 6h PER hs Chocolate Tortie Smoke Persian 6j PER js Lilac-Cream Smoke Persian 7 PER ns 21 Silver Tabby Persian 7a PER as 21 Blue Silver Tabby Persian 7b PER bs 21 Chocolate Silver Tabby Persian 7c PER cs 21 Lilac Silver Tabby Persian 7d PER ds 21 Red Silver Tabby Persian 7e PER fs 21 Tortie Silver Tabby Persian 7f PER es 21 Cream Silver Tabby Persian 7g PER gs 21 Blue Tortie Silver Tabby Persian 7h PER hs 21 Chocolate Tortie Silver Tabby Persian 7j PER js 21 Lilac Tortie Silver Tabby Persian 8 PER n 21 Brown Tabby Persian 8 30 PER n 24 Brown Spotted Tabby Persian 8 30a PER a 24 Blue Spotted Tabby Persian 8 38 PER n 24 Brown Spotted Tabby Persian 8 41 PER n 22 Brown Classic Tabby Persian 8 41a PER a 22 Blue Classic Tabby Persian 8 44 PER n 23 Brown Mackerel Tabby Persian 8 44a PER a 23 Blue Mackerel Tabby Persian 8 45 PER n 25 Brown Ticked Tabby Persian 8 45a PER a 25 Blue Ticked Tabby Persian 8a PER a 21 Blue Tabby Persian 8b PER b 21 Chocolate Tabby Persian 8c PER c 21 Lilac Tabby Persian 8e PER f 21 Tortie Tabby Persian 8f PER e 21 Cream Tabby Persian 8g -

THE GARDEN of ENGLAND CAT CLUB 2Nd & 3Rd December 2017
THE GARDEN OF ENGLAND CAT CLUB 2nd & 3rd December 2017 Present an International Cat Show According to FIFe Rules At Deanes Sport Centre Daws Heath Road, Benfleet, SS7 2TD - 1 - Felis Britannica Executive Committee President: Debbie Davies Vice President: Fran Williams General Secretary: Wendy Chapman Treasurer: John Tipper Deputy Treasurer: Mandy Rainbow Show Commission Chair: Tracey Milne Show Commission Debbie Davies Wendy Chapman Nick Chapman Breeding Health & Welfare Commission Chair: Shirley Watkinson Breeding Health & Welfare Commission Denise Fitten Marcia Morgan-Knight Registrar: Margaret Scott Rules Coordinator: Del Scott Publicity Officer: John Tipper Disciplinary Commission Kavita Vincent Webmaster: Stephanie Gower Felis Britannica Full Member Clubs Garden of England Cat Club – www.goecatclub.co.uk Viking Cat Club – www.vikingcatclub.co.uk The Scottish Cat Association – www.thescottishcatassociation.weebly.com General Information & Enquiries – [email protected] Garden of England online show entry – www.goecatclub.co.uk/entry membership – www.goecatclub.co.uk/online-membership membership renewal – www.goecatclub.co.uk/online-renewal On behalf of The Garden of England I would like to welcome you all to New Romney, we wish you all a very successful and enjoyable weekend - 2 - We are so lucky this weekend to have three well known International Judges with us: Kristof Van Roy –1B, 2, 3 & 4C (Belgium) Britta Busse – All Breed (Denmark) Stephe Bruin – All Breed (Netherlands) We also welcome student judge in categories 3 & 4 -
Genetic Assignment of Domestic Cats to Breeds and Worldwide Random-Bred Populations
doi: 10.1111/age.12008 Variation of cats under domestication: genetic assignment of domestic cats to breeds and worldwide random-bred populations J. D. Kurushima, M. J. Lipinski, B. Gandolfi, L. Froenicke, J. C. Grahn, R. A. Grahn and L. A. Lyons Department of Health & Reproduction, School of Veterinary Medicine, University of California – Davis, Davis, CA, 95616, USA. Summary Both cat breeders and the lay public have interests in the origins of their pets, not only in the genetic identity of the purebred individuals, but also in the historical origins of common household cats. The cat fancy is a relatively new institution with over 85% of its 40–50 breeds arising only in the past 75 years, primarily through selection on single-gene aesthetic traits. The short, yet intense cat breed history poses a significant challenge to the development of a genetic marker–based breed identification strategy. Using different breed assignment strategies and methods, 477 cats representing 29 fancy breeds were analysed with 38 short tandem repeats, 148 intergenic and five phenotypic single nucleotide polymorphisms. Results suggest the frequentist method of Paetkau (single nucleotide polymorphisms = 0.78, short tandem repeats = 0.88) surpasses the Bayesian method of Rannala and Mountain (single nucleotide polymorphisms = 0.56, short tandem repeats = 0.83) for accurate assignment of individuals to the correct breed. Additionally, a post-assignment verification step with the five phenotypic single nucleotide polymor- phisms accurately identified between 0.31 and 0.58 of the misassigned individuals raising the sensitivity of assignment with the frequentist method to 0.89 and 0.92 for single nucleotide polymorphisms and short tandem repeats respectively.