Worldwide Patterns in Mode of Development in Calyptraeid Gastropods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Crepidula Fornicata
NOBANIS - Marine invasive species in Nordic waters - Fact Sheet Crepidula fornicata Author of this species fact sheet: Kathe R. Jensen, Zoological Museum, Natural History Museum of Denmark, Universiteteparken 15, 2100 København Ø, Denmark. Phone: +45 353-21083, E-mail: [email protected] Bibliographical reference – how to cite this fact sheet: Jensen, Kathe R. (2010): NOBANIS – Invasive Alien Species Fact Sheet – Crepidula fornicata – From: Identification key to marine invasive species in Nordic waters – NOBANIS www.nobanis.org, Date of access x/x/201x. Species description Species name Crepidula fornicata, Linnaeus, 1758 – Slipper limpet Synonyms Patella fornicata Linnaeus, 1758; Crepidula densata Conrad, 1871; Crepidula virginica Conrad, 1871; Crepidula maculata Rigacci, 1866; Crepidula mexicana Rigacci, 1866; Crepidula violacea Rigacci, 1866; Crepidula roseae Petuch, 1991; Crypta nautarum Mörch, 1877; Crepidula nautiloides auct. NON Lesson, 1834 (ISSG Database; Minchin, 2008). Common names Tøffelsnegl (DK, NO), Slipper limpet, American slipper limpet, Common slipper snail (UK, USA), Østerspest (NO), Ostronpest (SE), Amerikanische Pantoffelschnecke (DE), La crépidule (FR), Muiltje (NL), Seba (E). Identification Crepidula fornicata usually sit in stacks on a hard substrate, e.g., a shell of other molluscs, boulders or rocky outcroppings. Up to 13 animals have been reported in one stack, but usually 4-6 animals are seen in a stack. Individual shells are up to about 60 mm in length, cap-shaped, distinctly longer than wide. The “spire” is almost invisible, slightly skewed to the right posterior end of the shell. Shell height is highly variable. Inside the body whorl is a characteristic calcareous shell-plate (septum) behind which the visceral mass is protected. -

Spinucella a New Genus of Miocene to Pleistocene , Muricid Gastropods from the Eastern Atlantic
Contr. Tert. Quatern. Geol. 30(1-2) 19-27 1 tab., 1 pi. Leiden, June 1993 Spinucella a new genus of Miocene to Pleistocene , muricid gastropods from the eastern Atlantic Geerat J. Vermeij University of California Davis, U.S.A. — new of Miocene Pleistocene muricid from the Atlantic. Contr. Tert. Vermeij, GeeratJ. Spinucella, a genus to gastropods eastern Quatern. Geol., 30(1-2): 19-27, 1 tab., 1 pi. Leiden, June 1993. The muricid is for de C. 1825 from the Pliocene of the new gastropod genus Spinucella proposed Purpura tetragonaJ. Sowerby, (type species), North Sea Basin, and for several other early Miocene to late Pleistocene species from southern Europe, North Africa, and southern Africa. The is characterised the of labral on the of the shell and reticulate of genus by presence a spine outer lip by sculpture. Species and Acanthinucella Cooke, 1918. The Spinucella closely resemble members ofNucella Röding, 1798, Acanthina Fischer von Waldheim, 1807, ofthat in the eastern Pacific Acanthina andAcanthinucella. Withthe removal of labral spine of Spinucellawas probably evolved independendy from where authors have the the time of arrival of Nucella in the North Atlantic from the S. tetragona Nucella, many recent placed species, North Pacific was late Pliocene, rather than middle Pliocene. — words Key Spinucella, new genus, Acanthina, Nucella, Neogene, biogeography. Prof. Dr G.J. Vermeij, Department of Geology, University of California, Davis, CA 95616, U.S.A. Contents 1956; Glibert, 1959, 1963). In fact, Glibert (1959) considered N. tetragona to be ancestral to N. lapillus, Introduction 19 p. the two species being linked by the late Pliocene 20 Systematics p. -

E Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary
!e Urban Sanctuary Algae and Marine Invertebrates of Ricketts Point Marine Sanctuary Jessica Reeves & John Buckeridge Published by: Greypath Productions Marine Care Ricketts Point PO Box 7356, Beaumaris 3193 Copyright © 2012 Marine Care Ricketts Point !is work is copyright. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced by any process without prior written permission of the publisher. Photographs remain copyright of the individual photographers listed. ISBN 978-0-9804483-5-1 Designed and typeset by Anthony Bright Edited by Alison Vaughan Printed by Hawker Brownlow Education Cheltenham, Victoria Cover photo: Rocky reef habitat at Ricketts Point Marine Sanctuary, David Reinhard Contents Introduction v Visiting the Sanctuary vii How to use this book viii Warning viii Habitat ix Depth x Distribution x Abundance xi Reference xi A note on nomenclature xii Acknowledgements xii Species descriptions 1 Algal key 116 Marine invertebrate key 116 Glossary 118 Further reading 120 Index 122 iii Figure 1: Ricketts Point Marine Sanctuary. !e intertidal zone rocky shore platform dominated by the brown alga Hormosira banksii. Photograph: John Buckeridge. iv Introduction Most Australians live near the sea – it is part of our national psyche. We exercise in it, explore it, relax by it, "sh in it – some even paint it – but most of us simply enjoy its changing modes and its fascinating beauty. Ricketts Point Marine Sanctuary comprises 115 hectares of protected marine environment, located o# Beaumaris in Melbourne’s southeast ("gs 1–2). !e sanctuary includes the coastal waters from Table Rock Point to Quiet Corner, from the high tide mark to approximately 400 metres o#shore. -

The American Slipper Limpet Crepidula Fornicata (L.) in the Northern Wadden Sea 70 Years After Its Introduction
Helgol Mar Res (2003) 57:27–33 DOI 10.1007/s10152-002-0119-x ORIGINAL ARTICLE D. W. Thieltges · M. Strasser · K. Reise The American slipper limpet Crepidula fornicata (L.) in the northern Wadden Sea 70 years after its introduction Received: 14 December 2001 / Accepted: 15 August 2001 / Published online: 25 September 2002 © Springer-Verlag and AWI 2002 Abstract In 1934 the American slipper limpet 1997). In the centre of its European distributional range Crepidula fornicata (L.) was first recorded in the north- a population explosion has been observed on the Atlantic ern Wadden Sea in the Sylt-Rømø basin, presumably im- coast of France, southern England and the southern ported with Dutch oysters in the preceding years. The Netherlands. This is well documented (reviewed by present account is the first investigation of the Crepidula Blanchard 1997) and sparked a variety of studies on the population since its early spread on the former oyster ecological and economic impacts of Crepidula. The eco- beds was studied in 1948. A field survey in 2000 re- logical impacts of Crepidula are manifold, and include vealed the greatest abundance of Crepidula in the inter- the following: tidal/subtidal transition zone on mussel (Mytilus edulis) (1) Accumulation of pseudofaeces and of fine sediment beds. Here, average abundance and biomass was 141 m–2 through the filtration activity of Crepidula and indi- and 30 g organic dry weight per square metre, respec- viduals protruding in stacks into the water column. tively. On tidal flats with regular and extended periods of This was reported to cause changes in sediments and emersion as well as in the subtidal with swift currents in near-bottom currents (Ehrhold et al. -

PUBLICATIONS 11 May 2021
ROBERT H. COWIE – PUBLICATIONS 11 May 2021 Google Scholar metrics Citations – 8939 (3794 since 2016), h-index – 47 (32 since 2016), i10-index – 109 (65 since 2016) Books (5) Joshi, R.C., Cowie, R.H. & Sebastian, L.S. (eds.) 2017. Biology and Management of Invasive Apple Snails. Philippine Rice Research Institute, Muñoz, Nueva Ecija. xvii + 405 p. Cowie, R.H., Rundell, R.J. & Yeung, N.W. 2017. Samoan Land Snails and Slugs – An Identification Guide. Department of Marine and Wildlife Resources, American Samoa Government. viii + 71 p. Cowie, R.[H.] 2014. Journey to a Waterfall. A Biologist in Africa. Lulu, Raleigh. x + 279 p. Staples, G.W. & Cowie, R.H. (eds.) 2001. Hawai‘i’s Invasive species. A guide to invasive plants and animals in the Hawaiian Islands. Mutual Publishing & Bishop Museum Press, Honolulu. xii + 116 p. Cowie, R.H., Evenhuis, N.L. & Christensen, C.C. 1995. Catalog of the native land and freshwater molluscs of the Hawaiian Islands. Backhuys Publishers, Leiden. vi + 248 p. Journal articles (136) 2021 Gerlach, J., Barker, G.M., Bick, C.S., Bouchet, P., Brodie, G., Christensen, C.C., Collins, T., Coote, T., Cowie, R.H., Fiedler, G.C., Griffiths, O.L., Florens, F.B.V, Hayes, K.A., Kim, J., Meyer, J.-Y., Meyer, W.M., III, Richling, I., Slapcinsky, J.D., Winsor, L. & Yeung, N.W. 2021. Negative impacts of the invasive predators Euglandina ‘rosea’ (Mollusca: Spiraxidae) and Platydemus manokwari (Platyhelminthes: Geoplanidae) when used as biological control agents against the pest snail Lisschatina fulica (Mollusca: Achatinidae). Biological Invasions 23(4): 997-1031. Rollins, R.L., Cowie, R.H., Echaluse, M.V. -

Download Preprint
1 Mobilising molluscan models and genomes in biology 2 Angus Davison1 and Maurine Neiman2 3 1. School of Life Sciences, University Park, University of Nottingham, NG7 2RD, UK 4 2. Department of Biology, University of Iowa, Iowa City, IA, USA and Department of Gender, 5 Women's, and Sexuality Studies, University of Iowa, Iowa, City, IA, USA 6 Abstract 7 Molluscs are amongst the most ancient, diverse, and important of all animal taxa. Even so, 8 no individual mollusc species has emerged as a broadly applied model system in biology. 9 We here make the case that both perceptual and methodological barriers have played a role 10 in the relative neglect of molluscs as research organisms. We then summarize the current 11 application and potential of molluscs and their genomes to address important questions in 12 animal biology, and the state of the field when it comes to the availability of resources such 13 as genome assemblies, cell lines, and other key elements necessary to mobilising the 14 development of molluscan model systems. We conclude by contending that a cohesive 15 research community that works together to elevate multiple molluscan systems to ‘model’ 16 status will create new opportunities in addressing basic and applied biological problems, 17 including general features of animal evolution. 18 Introduction 19 Molluscs are globally important as sources of food, calcium and pearls, and as vectors of 20 human disease. From an evolutionary perspective, molluscs are notable for their remarkable 21 diversity: originating over 500 million years ago, there are over 70,000 extant mollusc 22 species [1], with molluscs present in virtually every ecosystem. -

Crepidula Formicatacrepidula Formicata
Crepidula formicataCrepidula formicata Easily seen Crepidula fornicata along the shoreline. Class: Gastropoda Order: Littorinimorpha Family: Calyptraeidae Genus: Crepidula Distribution It is native to the eastern This species has been introduced to several other countries, coast of North America. especially in Europe. It is considered to be an invasive, nuisance It occurs from the Gulf of species. The first known occurrence of Crepidula fornicata in St. Lawrence in the north Europe was in 1872. It was introduced accidently with imported all the way south to the American oysters. They have been repeatedly introduced ever Gulf of Mexico. since in ballast water and on ships’ hulls. Habitat They are most abundant in muddy or mixed muddy areas, This species occupies a reaching their highest densities in wave protected locations. variety of seabed surfaces. These include bays, estuaries and sheltered sides of wave- They also occur in a wide exposed islands. In Nova Scotia they are common in intertidal range of environmental and shallow subtidal regions. They attach themselves to almost conditions. any hard surface to be found (including each other and the shells of other living or dead hard-shelled invertebrates. Food They are active suspension feeders, generating a water current They live off of plankton through the mantle cavity by ciliary (hair-like) action and which they filter out of the trapping food particles on a mucous sheet lying across the front seawater. surface of the gill filament. Reproduction Being hermaphrodites they contain both male and female The Slipper limpet is a. reproductive organs. Sperm producing ability advances quicker protandric hermaphrodite. -

ABSTRACT Title of Dissertation: PATTERNS IN
ABSTRACT Title of Dissertation: PATTERNS IN DIVERSITY AND DISTRIBUTION OF BENTHIC MOLLUSCS ALONG A DEPTH GRADIENT IN THE BAHAMAS Michael Joseph Dowgiallo, Doctor of Philosophy, 2004 Dissertation directed by: Professor Marjorie L. Reaka-Kudla Department of Biology, UMCP Species richness and abundance of benthic bivalve and gastropod molluscs was determined over a depth gradient of 5 - 244 m at Lee Stocking Island, Bahamas by deploying replicate benthic collectors at five sites at 5 m, 14 m, 46 m, 153 m, and 244 m for six months beginning in December 1993. A total of 773 individual molluscs comprising at least 72 taxa were retrieved from the collectors. Analysis of the molluscan fauna that colonized the collectors showed overwhelmingly higher abundance and diversity at the 5 m, 14 m, and 46 m sites as compared to the deeper sites at 153 m and 244 m. Irradiance, temperature, and habitat heterogeneity all declined with depth, coincident with declines in the abundance and diversity of the molluscs. Herbivorous modes of feeding predominated (52%) and carnivorous modes of feeding were common (44%) over the range of depths studied at Lee Stocking Island, but mode of feeding did not change significantly over depth. One bivalve and one gastropod species showed a significant decline in body size with increasing depth. Analysis of data for 960 species of gastropod molluscs from the Western Atlantic Gastropod Database of the Academy of Natural Sciences (ANS) that have ranges including the Bahamas showed a positive correlation between body size of species of gastropods and their geographic ranges. There was also a positive correlation between depth range and the size of the geographic range. -

Distribution, Abundance, and Diversity of Epifaunal Benthic Organisms in Alitak and Ugak Bays, Kodiak Island, Alaska
DISTRIBUTION, ABUNDANCE, AND DIVERSITY OF EPIFAUNAL BENTHIC ORGANISMS IN ALITAK AND UGAK BAYS, KODIAK ISLAND, ALASKA by Howard M. Feder and Stephen C. Jewett Institute of Marine Science University of Alaska Fairbanks, Alaska 99701 Final Report Outer Continental Shelf Environmental Assessment Program Research Unit 517 October 1977 279 We thank the following for assistance during this study: the crew of the MV Big Valley; Pete Jackson and James Blackburn of the Alaska Department of Fish and Game, Kodiak, for their assistance in a cooperative benthic trawl study; and University of Alaska Institute of Marine Science personnel Rosemary Hobson for assistance in data processing, Max Hoberg for shipboard assistance, and Nora Foster for taxonomic assistance. This study was funded by the Bureau of Land Management, Department of the Interior, through an interagency agreement with the National Oceanic and Atmospheric Administration, Department of Commerce, as part of the Alaska Outer Continental Shelf Environment Assessment Program (OCSEAP). SUMMARY OF OBJECTIVES, CONCLUSIONS, AND IMPLICATIONS WITH RESPECT TO OCS OIL AND GAS DEVELOPMENT Little is known about the biology of the invertebrate components of the shallow, nearshore benthos of the bays of Kodiak Island, and yet these components may be the ones most significantly affected by the impact of oil derived from offshore petroleum operations. Baseline information on species composition is essential before industrial activities take place in waters adjacent to Kodiak Island. It was the intent of this investigation to collect information on the composition, distribution, and biology of the epifaunal invertebrate components of two bays of Kodiak Island. The specific objectives of this study were: 1) A qualitative inventory of dominant benthic invertebrate epifaunal species within two study sites (Alitak and Ugak bays). -

A New Species of Bird's Nest Fungi: Characterisation of <I>Cyathus Subglobisporus</I>
Persoonia 21, 2008: 71–76 www.persoonia.org RESEARCH ARTICLE doi:10.3767/003158508X370578 A new species of bird’s nest fungi: characterisation of Cyathus subglobisporus sp. nov. based on morphological and molecular data R.-L. Zhao1, D.E. Desjardin 2, K. Soytong 3, K.D. Hyde 4, 5* Key words Abstract Recent collections of bird’s nest fungi (i.e. Crucibulum, Cyathus, Mycocalia, Nidula, and Nidularia species) in northern Thailand resulted in the discovery of a new species of Cyathus, herein described as C. subglobisporus. bird’s nest fungi This species is distinct by a combination of ivory-coloured fruiting bodies covered with shaggy hairs, plications on gasteromycetes the inner surface of the peridium and subglobose basidiospores. Phylogenetic analyses based on ITS and LSU new species ribosomal DNA sequences using neighbour-joining, maximum likelihood and weighted maximum parsimony sup- phylogeny port Cyathus subglobisporus as a distinct species and sister to a clade containing C. annulatus, C. renweii and rDNA C. stercoreus in the Striatum group. Article info Received: 24 June 2008; Accepted: 8 September 2008; Published: 23 September 2008. INTRODUCTION Cyathus striatus as representatives of the Nidulariaceae. Their phylogenetic reconstruction indicated that the Nidulariaceae The genus Cyathus along with the genera Crucibulum, Myco- was sister to the Cystodermateae (represented by Cystoderma calia, Nidula, and Nidularia are known as the bird’s nest fungi amianthinum). Together these two clades appear sister to the because of their small vase-shaped or nest-like fruiting bodies Agaricaceae s.l. but without bootstrap support. A phylogenetic containing lentil-shaped or egg-like peridioles. -

Rocky Shore Snails As Material for Projects (With a Key for Their Identification)
Field Studies, 10, (2003) 601 - 634 ROCKY SHORE SNAILS AS MATERIAL FOR PROJECTS (WITH A KEY FOR THEIR IDENTIFICATION) J. H. CROTHERS Egypt Cottage, Fair Cross, Washford, Watchet, Somerset TA23 0LY ABSTRACT Rocky sea shores are amongst the best habitats in which to carry out biological field projects. In that habitat, marine snails (prosobranchs) offer the most opportunities for individual investigations, being easy to find, to identify, to count and to measure and beng sufficiently robust to survive the experience. A key is provided for the identification of the larger species and suggestions are made for investigations to exploit selected features of individual species. INTRODUCTION Rocky sea shores offer one of the best habitats for individual or group investigations. Not only is there de facto public access (once you have got there) but also the physical factors that dominate the environment - tides (inundation versus desiccation), waves, heat, cold, light, dark, salinity etc. - change significantly over a few metres in distance. As a bonus, most of the fauna and flora lives out on the open rock surface and patterns of distribution may be clearly visible to the naked eye. Finally, they are amongst the most ‘natural’ of habitats in the British Isles; unless there has been an oil spill, rocky sea shores are unlikely to have been greatly affected by covert human activity. Some 270 species of marine snail (Phylum Mollusca, Class Gastropoda; Sub-Class Prosobranchia) live in the seas around the British Isles (Graham, 1988) and their empty shells may be found on many beaches. Most of these species are small (less than 3 mm long) or live beneath the tidemarks. -
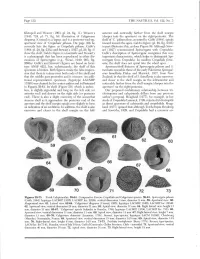
(1943: 724, Pi. 71, Fig. 16) Illustration of Calyptraea (Deeper Into the Aperture) on the Right/Posterior
Page 118 THE NAUTILUS, Vol. 122, No. 3 Kleinpeil and Weaver (1963, pi. 24, fig. 11). Weavers anterior and noticeably farther from the shell margin (1943: 724, pi. 71, fig. 16) illustration of Calyptraea (deeper into the aperture) on the right/posterior. The diegoana (Conrad) is a lapsus and is a posterior-end-up, shelf of 'C.y pileum thus, as noted by Gabb (1864), spirals apertural view of 'Crepidula' pileum. On page 356 he inward toward the apex. Gabb's figure (pi. 29, fig. 233b) correctly lists the figure as Crepidula pileum. Gabb's in part illustrates this, as does Figure 59. Although Stew- (1864: pi. 29, fig. 233a) and Stewart's (1927, pi. 29, fig. 3) art (1927) synonomized Spirocrypta with Crepidula, show the shelf. Gabb's figure is a fascimile and Stewart's Gabb's description of Spirocrypta recognizes this very is a photograph that has been reproduced in other dis- important characteristic, which helps to distinguish Spi- cussions of Spirocrypta (e.g., Wenz, 1940: 903, fig. rocrypta from Crepidula. In modern Crepidula forni- 2660a). Gabb's and Stewart's figures are based on lecto- cata, the shelf does not spiral into the whorl apex. type ANSP 4221, but, unfortunately, the shelf of this Aperture/shelf features of Spirocrypta pileum and S. specimen is broken. Both figures create the false impres- inomata resemble those of the early Paleocene Spirogal- sion that there is a sinus near both ends of the shelf and erus lamellaria Finlay and Marwick, 1937, from New that the middle part protrudes and is concave.