Gluten-Free-Diet Management/Coping
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Celiac Disease and Nonceliac Gluten Sensitivitya Review
Clinical Review & Education JAMA | Review Celiac Disease and Nonceliac Gluten Sensitivity A Review Maureen M. Leonard, MD, MMSc; Anna Sapone, MD, PhD; Carlo Catassi, MD, MPH; Alessio Fasano, MD CME Quiz at IMPORTANCE The prevalence of gluten-related disorders is rising, and increasing numbers of jamanetwork.com/learning individuals are empirically trying a gluten-free diet for a variety of signs and symptoms. This review aims to present current evidence regarding screening, diagnosis, and treatment for celiac disease and nonceliac gluten sensitivity. OBSERVATIONS Celiac disease is a gluten-induced immune-mediated enteropathy characterized by a specific genetic genotype (HLA-DQ2 and HLA-DQ8 genes) and autoantibodies (antitissue transglutaminase and antiendomysial). Although the inflammatory process specifically targets the intestinal mucosa, patients may present with gastrointestinal signs or symptoms, extraintestinal signs or symptoms, or both, Author Affiliations: Center for Celiac suggesting that celiac disease is a systemic disease. Nonceliac gluten sensitivity Research and Treatment, Division of is diagnosed in individuals who do not have celiac disease or wheat allergy but who Pediatric Gastroenterology and Nutrition, MassGeneral Hospital for have intestinal symptoms, extraintestinal symptoms, or both, related to ingestion Children, Boston, Massachusetts of gluten-containing grains, with symptomatic improvement on their withdrawal. The (Leonard, Sapone, Catassi, Fasano); clinical variability and the lack of validated biomarkers for nonceliac gluten sensitivity make Celiac Research Program, Harvard establishing the prevalence, reaching a diagnosis, and further study of this condition Medical School, Boston, Massachusetts (Leonard, Sapone, difficult. Nevertheless, it is possible to differentiate specific gluten-related disorders from Catassi, Fasano); Shire, Lexington, other conditions, based on currently available investigations and algorithms. -

Talent Boost Cookbook Finland
TALENT BOOST COOKBOOK FINLAND 1 Talent Boost Cookbook Finland A publication by Ministry of Economic Affairs and Employment and Authors: Business Finland. Pärtel-Peeter Pere Part of the Talent Boost programme of the Government of Finland. Marcus Andersson Morten King-Grubert Version 1.0 Research assistant: Leeni Vanhanen April 2019 at Future Place Leadership www.FuturePlaceLeadership.com An initiative of the Talent Boost national team: Laura Lindeman Visuals: Chief specialist at Ministry of Economic Affairs and Employment Finland Toolbox [email protected] https://toolbox.finland.fi/ Ulla Hiekkanen-Mäkelä Design by Design Agency Ruum 414: Head of Talent Boost Finland at Business Finland http://www.414.ee/ [email protected] #TalentBoost 2 EXECUTIVE SUMMARY ............................................................................................................................................................................................................ 4 INTRODUCTION ...................................................................................................................................................................................................................... 5 WHAT ARE THE INGREDIENTS OF AN ATTRACTIVE PLACE FOR TALENTS?.......................................................................................................................... 12 TALENT BOOST .................................................................................................................................................................................................................... -

Celiac Disease and Nonceliac Gluten Sensitivity a Review
Clinical Review & Education JAMA | Review Celiac Disease and Nonceliac Gluten Sensitivity A Review Maureen M. Leonard, MD, MMSc; Anna Sapone, MD, PhD; Carlo Catassi, MD, MPH; Alessio Fasano, MD CME Quiz at IMPORTANCE The prevalence of gluten-related disorders is rising, and increasing numbers of jamanetwork.com/learning individuals are empirically trying a gluten-free diet for a variety of signs and symptoms. This review aims to present current evidence regarding screening, diagnosis, and treatment for celiac disease and nonceliac gluten sensitivity. OBSERVATIONS Celiac disease is a gluten-induced immune-mediated enteropathy characterized by a specific genetic genotype (HLA-DQ2 and HLA-DQ8 genes) and autoantibodies (antitissue transglutaminase and antiendomysial). Although the inflammatory process specifically targets the intestinal mucosa, patients may present with gastrointestinal signs or symptoms, extraintestinal signs or symptoms, or both, Author Affiliations: Center for Celiac suggesting that celiac disease is a systemic disease. Nonceliac gluten sensitivity Research and Treatment, Division of is diagnosed in individuals who do not have celiac disease or wheat allergy but who Pediatric Gastroenterology and Nutrition, MassGeneral Hospital for have intestinal symptoms, extraintestinal symptoms, or both, related to ingestion Children, Boston, Massachusetts of gluten-containing grains, with symptomatic improvement on their withdrawal. The (Leonard, Sapone, Catassi, Fasano); clinical variability and the lack of validated biomarkers for nonceliac gluten sensitivity make Celiac Research Program, Harvard establishing the prevalence, reaching a diagnosis, and further study of this condition Medical School, Boston, Massachusetts (Leonard, Sapone, difficult. Nevertheless, it is possible to differentiate specific gluten-related disorders from Catassi, Fasano); Shire, Lexington, other conditions, based on currently available investigations and algorithms. -
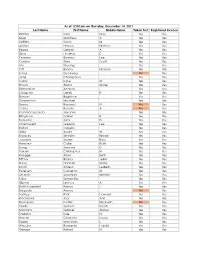
Last Name First Name Middle Name Taken Test Registered License
As of 12:00 am on Thursday, December 14, 2017 Last Name First Name Middle Name Taken Test Registered License Richter Sara May Yes Yes Silver Matthew A Yes Yes Griffiths Stacy M Yes Yes Archer Haylee Nichole Yes Yes Begay Delores A Yes Yes Gray Heather E Yes Yes Pearson Brianna Lee Yes Yes Conlon Tyler Scott Yes Yes Ma Shuang Yes Yes Ott Briana Nichole Yes Yes Liang Guopeng No Yes Jung Chang Gyo Yes Yes Carns Katie M Yes Yes Brooks Alana Marie Yes Yes Richardson Andrew Yes Yes Livingston Derek B Yes Yes Benson Brightstar Yes Yes Gowanlock Michael Yes Yes Denny Racheal N No Yes Crane Beverly A No Yes Paramo Saucedo Jovanny Yes Yes Bringham Darren R Yes Yes Torresdal Jack D Yes Yes Chenoweth Gregory Lee Yes Yes Bolton Isabella Yes Yes Miller Austin W Yes Yes Enriquez Jennifer Benise Yes Yes Jeplawy Joann Rose Yes Yes Harward Callie Ruth Yes Yes Saing Jasmine D Yes Yes Valasin Christopher N Yes Yes Roegge Alissa Beth Yes Yes Tiffany Briana Jekel Yes Yes Davis Hannah Marie Yes Yes Smith Amelia LesBeth Yes Yes Petersen Cameron M Yes Yes Chaplin Jeremiah Whittier Yes Yes Sabo Samantha Yes Yes Gipson Lindsey A Yes Yes Bath-Rosenfeld Robyn J Yes Yes Delgado Alonso No Yes Lackey Rick Howard Yes Yes Brockbank Taci Ann Yes Yes Thompson Kaitlyn Elizabeth No Yes Clarke Joshua Isaiah Yes Yes Montano Gabriel Alonzo Yes Yes England Kyle N Yes Yes Wiman Charlotte Louise Yes Yes Segay Marcinda L Yes Yes Wheeler Benjamin Harold Yes Yes George Robert N Yes Yes Wong Ann Jade Yes Yes Soder Adrienne B Yes Yes Bailey Lydia Noel Yes Yes Linner Tyler Dane Yes Yes -

The High German of Russian Mennonites in Ontario by Nikolai
The High German of Russian Mennonites in Ontario by Nikolai Penner A thesis presented to the University of Waterloo in fulfillment of the thesis requirement for the degree of Doctor of Philosophy in German Waterloo, Ontario, Canada, 2009 © Nikolai Penner 2009 Author’s Declaration I hereby declare that I am the sole author of this thesis. This is a true copy of the thesis, including any required final revisions, as accepted by examiners. I understand that my thesis may be made electronically available to the public. ii Abstract The main focus of this study is the High German language spoken by Russian Mennonites, one of the many groups of German-speaking immigrants in Canada. Although the primary language of most Russian Mennonites is a Low German variety called Plautdietsch, High German has been widely used in Russian Mennonite communities since the end of the eighteenth century and is perceived as one of their mother tongues. The primary objectives of the study are to investigate: 1) when, with whom, and for what purposes the major languages of Russian Mennonites were used by the members of the second and third migration waves (mid 1920s and 1940-50s respectively) and how the situation has changed today; 2) if there are any differences in spoken High German between representatives of the two groups and what these differences can be attributed to; 3) to what extent the High German of the subjects corresponds to the Standard High German. The primary thesis of this project is that different historical events as well as different social and political conditions witnessed by members of these groups both in Russia (e.g. -

Chester County Naturalization Records 1906-1935
Chester County Naturalization Records 1906-1935 Last Name First Name Middle Name Birth Place Date of Naturalization Date of Petition Book Page Petition # Date of Declaration Record of Declaration Sabatelli Antonio Italy 4 252 1146 June 5, 1919 Sabatelli Antonio Italy December 19, 1921 8 84 726 Sabatelli Giuseppe Italy 6 433 2319 October 6, 1924 Sabatini Francesco Italy 5 451 1843 January 30, 1923 Sabatini Francesco Italy January 30, 1923 13 26 1168 Sabatini Francesco Italy June 22, 1925 13 33 1175 Sabczynski Szczepan Russia Poland 3 104 503 May 19, 1915 Sabczynski Szczepan Russia June 26, 1922 9 49 791 Sabo Frank Hungary 9 3078 July 28, 1933 Sabo Frank Austria 3 383 781 February 12, 1917 Sabo Franz Austria Hungary December 18, 1922 9 94 836 Sabo George Austria Hungary 5 150 1542 March 29, 1921 Sabo John Czechoslovakia December 17, 1928 16 14 1756 Sabo Joseph Hungary 9 2950 August 9, 1930 Sabo Joseph Hungary June 24, 1935 19 2138 Sabo Steve Czechoslovakia March 16, 1925 13 60 1202 Sabol Frank Hungary 7 16 2402 March 24, 1925 Sabol Frank Hungary March 26, 1928 15 72 1564 Sabol John Hungary 3 12 411 December 1, 1913 Sabol John Hungary March 13, 1916 4 20 270 Sabol Majk Hungary 1 series 249 49 January 27, 1908 Saboucsik Michal Czechoslovakia December 19, 1927 15 115 1607 Chester County Archives and Record Services, West Chester, PA 19380 Last Name First Name Middle Name Birth Place Date of Naturalization Date of Petition Book Page Petition # Date of Declaration Record of Declaration Sacco Alex Italy 5 210 1602 September 16, 1921 Sacco Alexander -

Surname First Name Categorisation Abadin Jose Luis Silver Abbelen
2018 DRIVERS' CATEGORISATION LIST Updated on 09/07/2018 Drivers in red : revised categorisation Drivers in blue : new categorisation Surname First name Categorisation Abadin Jose Luis Silver Abbelen Klaus Bronze Abbott Hunter Silver Abbott James Silver Abe Kenji Bronze Abelli Julien Silver Abergel Gabriele Bronze Abkhazava Shota Bronze Abra Richard Silver Abreu Attila Gold Abril Vincent Gold Abt Christian Silver Abt Daniel Gold Accary Thomas Silver Acosta Hinojosa Julio Sebastian Silver Adam Jonathan Platinum Adams Rudi Bronze Adorf Dirk Silver Aeberhard Juerg Silver Afanasiev Sergei Silver Agostini Riccardo Gold Aguas Rui Gold Ahlin-Kottulinsky Mikaela Silver Ahrabian Darius Bronze Ajlani Karim Bronze Akata Emin Bronze Aksenov Stanislas Silver Al Faisal Abdulaziz Silver Al Harthy Ahmad Silver Al Masaood Humaid Bronze Al Qubaisi Khaled Bronze Al-Azhari Karim Bronze Alberico Neil Silver Albers Christijan Platinum Albert Michael Silver Albuquerque Filipe Platinum Alder Brian Silver Aleshin Mikhail Platinum Alesi Giuliano Silver Alessi Diego Silver Alexander Iradj Silver Alfaisal Saud Bronze Alguersuari Jaime Platinum Allegretta Vincent Silver Alleman Cyndie Silver Allemann Daniel Bronze Allen James Silver Allgàuer Egon Bronze Allison Austin Bronze Allmendinger AJ Gold Allos Manhal Bronze Almehairi Saeed Silver Almond Michael Silver Almudhaf Khaled Bronze Alon Robert Silver Alonso Fernando Platinum Altenburg Jeff Bronze Altevogt Peter Bronze Al-Thani Abdulrahman Silver Altoè Giacomo Silver Aluko Kolawole Bronze Alvarez Juan Cruz Silver Alzen -

Kierkegaard, Literature, and the Arts
Kierke gaard, Literature, and the Arts Engraving, ca. 1837, by Carl Strahlheim showing the Gendarmenmarkt in Berlin, with what was then the Schauspielhaus, or Theater (center)— now the concert house of the Konzerthausorchester Berlin— flanked by the German Cathedral (left) and the French Cathedral (right). Pictured in the background to the immediate right of the theater is the building, still standing today, in which Kierkegaard lodged during his four stays in Berlin, in 1841– 42, 1843, 1845, and 1846. It was there, as noted by a plaque outside, that Kierkegaard wrote the first drafts of Either/Or, Repetition, and Fear and Trembling. Kierkegaard, Literature, and the Arts Edited by Eric Ziolkowski northwestern university press evanston, illinois Northwestern University Press www.nupress.northwestern.edu Copyright © 2018 by Northwestern University Press. Published 2018. All rights reserved. Printed in the United States of America 10 9 8 7 6 5 4 3 2 1 Library of Congress Cataloging- in- Publication Data Names: Ziolkowski, Eric Jozef, 1958– editor. Title: Kierkegaard, literature, and the arts / edited by Eric Ziolkowski. Description: Evanston, Illinois : Northwestern University Press, 2018. | Includes index. Identifiers: LCCN 2017029795 | ISBN 9780810135970 (cloth : alk. paper) | ISBN 9780810135963 (pbk. : alk. paper) | ISBN 9780810135987 (e-book) Subjects: LCSH: Kierkegaard, Søren, 1813–1855. | Kierkegaard, Søren, 1813– 1855—Aesthetics. | Literature—Philosophy. | Music and philosophy. | Art and philosophy. | Performing arts—Philosophy. Classification: LCC B4377 .K4558 2018 | DDC 198.9—dc23 LC record available at https://lccn.loc.gov/2017029795 Except where otherwise noted, this book is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. -
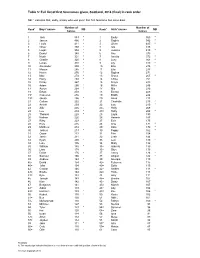
Table 5: Full List of First Forenames Given, Scotland, 2014 (Final) in Rank Order
Table 5: Full list of first forenames given, Scotland, 2014 (final) in rank order NB: * indicates that, sadly, a baby who was given that first forename has since died. Number of Number of Rank1 Boys' names NB Rank1 Girls' names NB babies babies 1 Jack 583 * 1 Emily 569 * 2 James 466 * 2 Sophie 542 * 3 Lewis 411 * 3 Olivia 485 * 4 Oliver 403 * 4 Isla 435 5 Logan 354 * 5 Jessica 419 * 6 Daniel 349 * 6 Ava 379 7 Noah 321 * 7 Amelia 372 * 8 Charlie 320 * 8 Lucy 363 * 9 Lucas 310 * 9 Lily 310 * 10 Alexander 309 * 10 Ellie 278 * 11 Mason 285 * 11 Ella 273 12 Harris 276 * 12 Sophia 271 13 Max 274 * 13 Grace 265 * 14 Harry 268 * 14 Chloe 251 15 Finlay 267 15 Freya 249 16 Adam 266 * 16 Millie 246 17 Aaron 264 * 17 Mia 230 18 Ethan 259 18 Emma 229 19= Cameron 256 19 Eilidh 224 19= Jacob 256 * 20 Anna 218 21 Callum 252 21 Charlotte 213 * 22 Archie 239 22 Eva 210 * 23 Alfie 236 * 23= Holly 208 24 Leo 234 * 23= Ruby 208 * 25 Thomas 228 * 25 Layla 190 26 Nathan 226 26 Hannah 187 27 Riley 223 * 27 Evie 175 28 Rory 215 28 Orla 171 * 29 Matthew 214 29 Katie 170 * 30 Joshua 213 * 30 Poppy 162 * 31 Oscar 212 31 Erin 154 32 Jamie 211 32 Leah 144 33 Ryan 208 * 33 Lexi 140 * 34 Luke 195 34 Molly 132 35 William 180 * 35= Isabella 130 36 Liam 178 35= Skye 130 37 Dylan 176 * 37 Lacey 124 * 38 Samuel 166 38 Abigail 122 * 39 Andrew 163 * 39 Georgia 119 40= David 154 * 40= Rebecca 115 40= John 154 40= Sofia 115 42 Connor 146 42= Amber 113 * 43= Brodie 144 42= Hollie 113 43= Kyle 144 * 44 Amy 111 45 Joseph 143 45= Brooke 107 46 Kian 142 * 45= Daisy 107 47 Benjamin 141 45= Niamh 107 48= Aiden 139 48= Lilly 106 48= Harrison 139 * 48= Zoe 106 * 50 Robert 135 * 50 Rosie 102 51= Ben 130 51 Abbie 101 51= Muhammad 130 52 Robyn 100 * 53 Michael 127 53 Sienna 98 54 Tyler 123 * 54= Summer 96 55 Kai 120 54= Zara 96 56 Euan 114 * 56 Iona 91 57= Arran 112 57 Maya 89 57= Jayden 112 58 Sarah 88 59 Jake 111 * 59= Aria 87 60= Cole 110 59= Maisie 87 60= Ollie 110 * 61 Cara 85 Footnote 1) The equals sign indicates that there is more than one name with this position of rank in the list. -

Finland, the Observatoryisapartnership,Hostedbywho/Europe, Organizations International Whichincludesother
V ol. 21 Health Systems in Transition Vol. 21 No. 2 2019 No. 2 0 1 9 Heal t h S y s te m s in T r an s ition: Finland Finland Health system review Ilmo Keskimäki Vesa Syrjä Liina-Kaisa Tynkkynen Lauri Vuorenkoski Eeva Reissell Bernd Rechel Meri Koivusalo Marina Karanikolos The Observatory is a partnership, hosted by WHO/Europe, which includes other international organizations (the European Commission, the World Bank); national and regional governments (Austria, Belgium, Finland, Ireland, Norway, Slovenia, Spain, Sweden, Switzerland, the United Kingdom and the Veneto Region of Italy); other health system organizations (the French National Union of Health Insurance Funds (UNCAM), the Health Foundation); and academia (the London School of Economics and Political Science (LSE) and the London School of Hygiene & Tropical Medicine (LSHTM)). The Observatory has a secretariat in Brussels and it has hubs in London at LSE and LSHTM) and at the Berlin University of Technology. HiTs are in-depth profiles of health systems and policies, produced using a standardized approach that allows comparison across countries. They provide facts, figures and analysis and highlight reform initiatives in progress. Print ISSN 1817-6119 Web ISSN 1817-6127 61546 Finland HiT_covers_WEB.pdf 2 02/09/2019 14:15 Marina Karanikolos and Bernd Rechel (Editors), and Ewout van Ginneken (Series editor) were responsible for this HiT Editorial Board Series editors Reinhard Busse, Berlin University of Technology, Germany Josep Figueras, European Observatory on Health Systems and -

Alcohol Marketing on Social Media Sites in Finland And
113 113 2019 ALCOHOL MARKETING ON SOCIAL MEDIA SITES IN FINLAND SITES MARKETING MEDIA ON AND SOCIAL SWEDEN ALCOHOL Emmi Kauppila, Mikaela Lindeman, Johan Svensson, Matilda Hellman and Anu Katainen ALCOHOL MARKETING ON SOCIAL MEDIA SITES IN FINLAND AND SWEDEN A comparative audit study of brands’ presence and content, and the impact of a legislative change ISSN 2343-273X ISBN 978-951-51-3381-6 Publications of the Faculty of Social Sciences of the Faculty Publications Faculty of Social Sciences University of Helsinki Finland Alcohol marketing on social media sites in Finland and Sweden A comparative audit study of brands’ presence and content, and the impact of a legislative change Emmi Kauppila, Mikaela Lindeman, Johan Svensson, Matilda Hellman and Anu Katainen REPORT University of Helsinki Centre for Research on Addiction, Control and Governance (CEACG) Helsinki 2019 Publications of the Faculty of Social Sciences 113 (2019) Cover picture: Elias Wulff, 2019 ISSN 2343-273X (print) ISSN 2343-2748 (online) ISBN 978-951-51-3381-6 (pbk) ISBN 978-951-51-3382-3 (PDF) Unigrafia Helsinki 2019 Abstract Social media has become a key marketing platform for alcohol brands. Social media makes it possible for advertisers to spread messages via consumers and to involve them in the production of marketing content. It offers new possibilities for interactive communication between alcoholic beverage companies and their potential consumers. This report presents the first audit of alcoholic beverage brands’ activities on social media targeting consumers in Finland and Sweden. Its purpose is to produce new information on how this issue can be viewed as a marketing effort, what kind of content is used, and how well alcohol producers have succeeded in reaching consumers on their social media channels. -

Service Number Veterans Names Veterans Surname Name Veterans
Veterans Surname Service Number Veterans Names Veterans Given Names Column-In/Outside Row Enlistment Name H6960 Abgrall, Harvey Abgrall Harvey 6-I 50 WWII 1001003 Abrahamson, Clarence Franklin Abrahamson Clarence Franklin 6-O 41 WWI L86912 Abram, Albert Joseph Abram Albert Joseph 3-I 13 WWII H62998 Ackabee, Stanley Michael Joseph Ackabee Stanley Michael Joseph 3-O 16 WWII H40755 Adam, Robert Nesbit Adam Robert Nesbit 5-O 22 WWII J21975 Adam, Thomas Wilkie Adam Thomas Wilkie 3-O 13 WWII Adams, A.J. Adams A.J. 2-I 55 WWII M34080 Adams, C.H. Adams C.H. 2-I 21 WWII Adams, Cecil George Adams Cecil George 7-O 30 WWII B98233 Adams, Clarence Adams Clarence 4-I 70 WWII Adams, Claude A. Adams Claude A. 4-I 2 WWII Adams, Ernest Gordon Adams Ernest Gordon 8-O 51 WWII H6502 Adams, Frederick George Adams Frederick George 4-O 26 WWII Adams, George Stanley Adams George Stanley 4-I 18 WWII Adams, Glen Adams Glen 4-I 71 WWII Adams, Gordon Adams Gordon 5-I 9 WWII L100759 Adams, Homer Adams Homer 3-O 14 WWII H6294 Adams, John Henry Adams John Henry 5-I 44 WWII H6402 Adams, L. G. Adams L. G. 6-I 30 WWII Adams, Leonard Adams Leonard 8-O 41 WWII Adams, Leslie William Adams Leslie William 8-O 14 WWII Adams, Lewis Henry Adams Lewis Henry 3-I 53 WWII P22233 Adams, Lloyd Herman Adams Lloyd Herman 2-I 36 WWII H6628 Adams, R. D. Adams R. D. 5-I 41 WWII Adams, Sidney Alexander Armstrong Adams Sidney Alexander Armstrong 1-I 12 WWII 504471 Adams, Sidney Harold Adams Sidney Harold 5-I 26 WWI Adams, Stanley Adams Stanley 5-I 13 WWII P22318 Adams, Stanley Adams Stanley 8-O 67 WWII Adams, Thomas Leslie Adams Thomas Leslie 5-O 53 WWII Veterans Surname Service Number Veterans Names Veterans Given Names Column-In/Outside Row Enlistment Name Adams, W.