COVID-19 Vaccine Planning and Considerations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

What Do We Know About India's Covaxin Vaccine?
FEATURE Tamil Nadu, India COVID-19 VACCINES [email protected] BMJ: first published as 10.1136/bmj.n997 on 20 April 2021. Downloaded from Cite this as: BMJ 2021;373:n997 http://dx.doi.org/10.1136/bmj.n997 What do we know about India’s Covaxin vaccine? Published: 20 April 2021 India has rapidly approved and rolled out Covaxin, its own covid-19 vaccine. Kamala Thiagarajan examines what we know so far. Kamala Thiagarajan freelance journalist Who developed Covaxin? cheapest purchased by any country in the world at 206 rupees per shot for the 5.5 million doses the Covaxin was developed by Indian pharmaceutical government currently has on order. The government company Bharat Biotech in collaboration with the has capped the price of the vaccine sold in the private Indian Council of Medical Research, a government market, with private hospitals able to charge up to funded biomedical research institute, and its 250 rupees.13 subsidiary the National Institute of Virology. Covaxin does not require storage at sub-zero Bharat Biotech has brought to market 16 original temperatures, which would be hard to maintain in vaccines, including for rotavirus, hepatitis B, Zika India’s climate and with the frequent power cuts in virus, and chikungunya.1 The company reportedly rural areas. Covaxin is available in multi-dose vials spent $60-$70m (£43-£50m; €50-€58m) developing and is stable at the 2-8°C that ordinary refrigeration Covaxin.2 can achieve. How does Covaxin work? Bharat Biotech says it has a stockpile of 20 million The vaccine is similar to CoronaVac (the Chinese doses of Covaxin for India and is in the process of vaccine developed by Sinovac)3 in that it uses a manufacturing 700 million doses at its four facilities complete infective SARS-CoV-2 viral particle in two cities by the end of the year. -

The Effectiveness of Non-Pharmaceutical Interventions In
medRxiv preprint doi: https://doi.org/10.1101/2020.04.06.20054197; this version posted April 10, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . The effectiveness of non-pharmaceutical interventions in containing epidemics: a rapid review of the literature and quantitative assessment CHEATLEY Jane1, VUIK Sabine1, DEVAUX Marion1, SCARPETTA Stefano1, PEARSON Mark1, COLOMBO Francesca1, CECCHINI Michele1 1. Directorate for Employment, Labour and Social Affairs, Organization for Economic Co-operation and Development, Paris, France Corresponding author: CECCHINI Michele: [email protected] Abstract The number of confirmed COVID-19 cases has rapidly increased since discovery of the disease in December 2019. In the absence of medical countermeasures to stop the spread of the disease (i.e. vaccines), countries have responded by implementing a suite of non-pharmaceutical interventions (NPIs) to contain and mitigate COVID-19. Individual NPIs range in intensity (e.g. from lockdown to public health campaigns on personal hygiene), as does their impact on reducing disease transmission. This study uses a rapid review approach and investigates evidence from previous epidemic outbreaks to provide a quantitative assessment of the effectiveness of key NPIs used by countries to combat the COVID-19 pandemic. Results from the study are designed to help countries enhance their policy response as well as inform transition strategies by identifying which policies should be relaxed and which should not. Introduction In December 2019, Wuhan, located in the Hubei province of China, experienced an outbreak of pneumonia from a novel virus – severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Chen et al., 2020[1]). -

COVID-19 Vaccines Update Supplement Week Of: 5Th April, 2021
CARPHA UPDATE FOR Incident Manager / SITUATION REPORT COVID-19 Vaccines Update Supplement Week of: 5th April, 2021 I. Overview of Development and Regulatory Approvals: • 85 candidate vaccines are in clinical development: 16 in Phase 3 trials, and 4 in Phase 4 trials – see Figure in CARPHA COVID-19 Vaccine Regulatory Tracker (Phases tab). • 13 vaccines have received regulatory approvals in various countries, and 16 vaccines are at various stages of engagement with WHO for emergency use listing (EUL). • 4 vaccines have been approved by WHO for Emergency Use Listing: Pfizer-BioNTech’s vaccine: COMIRNATY®, AstraZeneca-SK Bio, AstraZeneca-SII (Covishield), and Janssen-Cilag. • 4 additional vaccines are expected to be approved by WHO in April – Tables 1 and 3. • There are 3 additional vaccines being considered by WHO but these are at the stage of submitting expressions of interest: Bharat Biotech, Clover Biopharmaceuticals-GSK + Dynavax, and BioCubaFarma (Cuba). • The WHO Strategic Advisory Group of Experts on Immunization (SAGE) interim recommendations and background documents are available for vaccines by: Moderna, Pfizer-BioNTech, AstraZeneca-Oxford and Janssen-Cilag at SAGE Interim Guidance. The recommendations provide guidance on the use the vaccines, including use in various groups. • Reports of rare clotting events among vaccinees continue to be assessed by various regulators. The EU's European Medicines Agency (EMA) has stated that there was no evidence to support decisions by regulators to restrict the use of Oxford-AstraZeneca vaccines in some age groups. The WHO maintains that the benefit-risk balance of the vaccine remains favorable. • CARPHA has shared its COVID-19 vaccine regulatory tracker with Member States for viewing as updates are made. -

COVID-19 Vaccines: Update on Allergic Reactions, Contraindications, and Precautions
Centers for Disease Control and Prevention Center for Preparedness and Response COVID-19 Vaccines: Update on Allergic Reactions, Contraindications, and Precautions Clinician Outreach and Communication Activity (COCA) Webinar Wednesday, December 30, 2020 Continuing Education Continuing education will not be offered for this COCA Call. To Ask a Question ▪ All participants joining us today are in listen-only mode. ▪ Using the Webinar System – Click the “Q&A” button. – Type your question in the “Q&A” box. – Submit your question. ▪ The video recording of this COCA Call will be posted at https://emergency.cdc.gov/coca/calls/2020/callinfo_123020.asp and available to view on-demand a few hours after the call ends. ▪ If you are a patient, please refer your questions to your healthcare provider. ▪ For media questions, please contact CDC Media Relations at 404-639-3286, or send an email to [email protected]. Centers for Disease Control and Prevention Center for Preparedness and Response Today’s First Presenter Tom Shimabukuro, MD, MPH, MBA CAPT, U.S. Public Health Service Vaccine Safety Team Lead COVID-19 Response Centers for Disease Control and Prevention Centers for Disease Control and Prevention Center for Preparedness and Response Today’s Second Presenter Sarah Mbaeyi, MD, MPH CDR, U.S. Public Health Service Clinical Guidelines Team COVID-19 Response Centers for Disease Control and Prevention National Center for Immunization & Respiratory Diseases Anaphylaxis following mRNA COVID-19 vaccination Tom Shimabukuro, MD, MPH, MBA CDC COVID-19 Vaccine -

V.3 1/25/2021 COVID-19 Vaccine FAQ Sheet
COVID-19 Vaccine FAQ Sheet (updated 1/25/2021) The AST has received queries from transplant professionals and the community regarding the COVID-19 vaccine. The following FAQ was developed to relay information on the current state of knowledge. This document is subject to change and will be updated frequently as new information or data becomes available. What kinds of vaccines are available or under development to prevent COVID-19? There are currently several vaccine candidates in use or under development. In the United States, the Government is supporting six separate vaccine candidates. Several other vaccines are also undergoing development outside of the United States government sponsorship and further information can be found here: • NYTimes Coronavirus Vaccine Tracker: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine- tracker.html • Washington Post Vaccine Tracker: https://www.washingtonpost.com/graphics/2020/health/covid-vaccine-update- coronavirus/ The types of vaccines are as follows (January 25, 2021) 1: Table 1: Vaccines Under Development Vaccine Type Compound Name Clinical Trial Notes [Sponsor] Phase mRNA mRNA-1273 Phase 3 Emergency use in [Moderna] U.S., E.U., other countries Approved in Canada BNT162b2 [Pfizer] Phase 2/3 Emergency use in U.S., E.U., other countries Also approved in Canada and other countries Replication- AZD1222 Phase 2/3 Emergency use in defective [AstraZeneca] Britain, India, other adenoviral vector countries (not U.S.) Ad26.COV2.S Phase 3 [Janssen] v.3 1/25/2021 Recombinant- NVX-CoV2373 Phase 3 subunit-adjuvanted [Novavax] protein Recombinant SARS- Phase 1/2 CoV-2 Protein Antigen + AS03 Adjuvant [Sanofi Pasteur/GSK] Inactivated CoVaxin [Bharat Phase 3 Emergency Use in coronavirus Biotech] India BBIBP-CorV Phase 3 Approved China, [Sinopharm] Bahrain, UAE; Emergency use elsewhere (not U.S.) Both of the mRNA SARS-CoV-2 vaccines (Moderna, Pfizer) have been approved by Emergency Use Authorization by the U.S. -

COVID-19 Vaccination Programme: Information for Healthcare Practitioners
COVID-19 vaccination programme Information for healthcare practitioners Republished 6 August 2021 Version 3.10 1 COVID-19 vaccination programme: Information for healthcare practitioners Document information This document was originally published provisionally, ahead of authorisation of any COVID-19 vaccine in the UK, to provide information to those involved in the COVID-19 national vaccination programme before it began in December 2020. Following authorisation for temporary supply by the UK Department of Health and Social Care and the Medicines and Healthcare products Regulatory Agency being given to the COVID-19 Vaccine Pfizer BioNTech on 2 December 2020, the COVID-19 Vaccine AstraZeneca on 30 December 2020 and the COVID-19 Vaccine Moderna on 8 January 2021, this document has been updated to provide specific information about the storage and preparation of these vaccines. Information about any other COVID-19 vaccines which are given regulatory approval will be added when this occurs. The information in this document was correct at time of publication. As COVID-19 is an evolving disease, much is still being learned about both the disease and the vaccines which have been developed to prevent it. For this reason, some information may change. Updates will be made to this document as new information becomes available. Please use the online version to ensure you are accessing the latest version. 2 COVID-19 vaccination programme: Information for healthcare practitioners Document revision information Version Details Date number 1.0 Document created 27 November 2020 2.0 Vaccine specific information about the COVID-19 mRNA 4 Vaccine BNT162b2 (Pfizer BioNTech) added December 2020 2.1 1. -
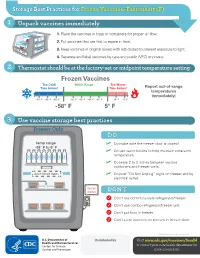
Storage Best Practices for Frozen Vaccines-Fahrenheit
Storage Best Practices for Frozen Vaccines–Fahrenheit (F) 1 Unpack vaccines immediately 1. Place the vaccines in trays or containers for proper air flow. 2. Put vaccines that are first to expire in front. HEP A - VFC 3. Keep vaccines in original boxes with lids closed to prevent exposure to light. 4. Separate and label vaccines by type and public (VFC) or private. 2 Thermostat should be at the factory-set or midpoint temperature setting Frozen Vaccines Too Cold! Within Range Too Warm! Take Action! Take Action! Report out-of-range temperatures immediately! -70° F -65° F -60° F -50° F -45° F -40° F -35° F 10° F 15° F -58° F 5° F 3 Use vaccine storage best practices Freezer Only DO temp range ✓ Do make sure the freezer door is closed! -58° F to 5° F ✓ Do use water bottles to help maintain consistent temperature. ✓ Do leave 2 to 3 inches between vaccine containers and freezer walls. don’t block vents ✓ Do post “Do Not Unplug” signs on freezer and by electrical outlet. do not unplug DON’T Don’t use dormitory-style refrigerator/freezer. Don’t use combo refrigerator/freezer unit. Don’t put food in freezer. Don’t store vaccines on shelves in freezer door. CS243541-D Revision December 2020 Distributed by Visit www.cdc.gov/vaccines/SandH or contact your state health department for more information. Test Your Knowledge 1 Which of the following units is the best for storing frozen vaccines? Freezer Freezer Freezer Freezer A. Full-size B. Full-size C. Stand-alone D. -

Considerations for Causality Assessment of Neurological And
Occasional essay J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp-2021-326924 on 6 August 2021. Downloaded from Considerations for causality assessment of neurological and neuropsychiatric complications of SARS- CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder Matt Butler ,1 Arina Tamborska,2,3 Greta K Wood,2,3 Mark Ellul,4 Rhys H Thomas,5,6 Ian Galea ,7 Sarah Pett,8 Bhagteshwar Singh,3 Tom Solomon,4 Thomas Arthur Pollak,9 Benedict D Michael,2,3 Timothy R Nicholson10 For numbered affiliations see INTRODUCTION More severe potential adverse effects in the open- end of article. The scientific community rapidly responded to label phase of vaccine roll- outs are being collected the COVID-19 pandemic by developing novel through national surveillance systems. In the USA, Correspondence to SARS- CoV-2 vaccines (table 1). As of early June Dr Timothy R Nicholson, King’s roughly 372 adverse events have been reported per College London, London WC2R 2021, an estimated 2 billion doses have been million doses, which is a lower rate than expected 1 2LS, UK; timothy. nicholson@ administered worldwide. Neurological adverse based on the clinical trials.6 kcl. ac. uk events following immunisation (AEFI), such as In the UK, adverse events are reported via the cerebral venous sinus thrombosis and demyelin- MB and AT are joint first Coronavirus Yellow Card reporting website. As of ating episodes, have been reported. In some coun- authors. early June 2021, approximately 250 000 Yellow tries, these have led to the temporary halting of BDM and TRN are joint senior Cards have been submitted, equating to around authors. -

Electronic Support for Public Health–Vaccine Adverse Event Reporting System (ESP:VAERS)
Grant Final Report Grant ID: R18 HS 017045 Electronic Support for Public Health–Vaccine Adverse Event Reporting System (ESP:VAERS) Inclusive dates: 12/01/07 - 09/30/10 Principal Investigator: Lazarus, Ross, MBBS, MPH, MMed, GDCompSci Team members: Michael Klompas, MD, MPH Performing Organization: Harvard Pilgrim Health Care, Inc. Project Officer: Steve Bernstein Submitted to: The Agency for Healthcare Research and Quality (AHRQ) U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov Abstract Purpose: To develop and disseminate HIT evidence and evidence-based tools to improve healthcare decision making through the use of integrated data and knowledge management. Scope: To create a generalizable system to facilitate detection and clinician reporting of vaccine adverse events, in order to improve the safety of national vaccination programs. Methods: Electronic medical records available from all ambulatory care encounters in a large multi-specialty practice were used. Every patient receiving a vaccine was automatically identified, and for the next 30 days, their health care diagnostic codes, laboratory tests, and medication prescriptions were evaluated for values suggestive of an adverse event. Results: Restructuring at CDC and consequent delays in terms of decision making have made it challenging despite best efforts to move forward with discussions regarding the evaluation of ESP:VAERS performance in a randomized trial and comparison of ESP:VAERS performance to existing VAERS and Vaccine Safety Datalink data. However, Preliminary data were collected and analyzed and this initiative has been presented at a number of national symposia. Key Words: electronic health records, vaccinations, adverse event reporting The authors of this report are responsible for its content. -

CDC Vaccine Storage and Handling Guide
Table of Contents General Information Vaccine Storage and Handling Best Practices 5 Selected Biologicals Diphtheria Toxoid-, Tetanus Toxoid- and acellular Pertussis-Containing Vaccines DTaP: DAPTACEL, Infanrix, Tripedia 11 DTaP-IPV: KINRIX 11 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV/Hib: Pentacel 11 Haemophilus influenzae type b-Containing Vaccines Hib: ActHIB, Hiberix, PedvaxHIB 15 Hib-HepB: Comvax 15 DTaP-IPV/Hib: Pentacel 11 Hepatitis-Containing Vaccines HepA: Havrix, VAQTA 19 HepB: Engerix-B, Recombivax HB 19 HepA-HepB: Twinrix 19 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-HepB-IPV: Pediarix 11 Hib-HepB: Comvax 15 Human Papillomavirus Vaccines HPV2: Cervarix 23 HPV4: Gardasil 23 Vaccine Storage and Handling Guide —————————————————————————————— Page 2 Table of Contents Influenza Vaccines LAIV: FluMist 27 TIV: Afluria, Fluarix, FluLaval, Fluvirin, Fluzone, Fluzone High-Dose, Fluzone Intradermal 29 Measles-, Mumps- and Rubella-Containing Vaccine MMR: M-M-RII 33 MMRV: ProQuad 69 Meningococcal Vaccines MCV4: Menactra, Menveo 37 MPSV4: Menomune 41 Pneumococcal Vaccines PCV13: Prevnar 13 45 PPSV23: Pneumovax 23 45 Poliovirus-Containing Vaccine IPV: IPOL 49 DTaP-HepB-IPV: Pediarix 11 DTaP-IPV: KINRIX 11 Vaccine Storage and Handling Guide and Storage Vaccine National Center for Immunization and Respiratory Disease DTaP-IPV/Hib: Pentacel 11 Rotavirus Vaccines RV1: ROTARIX 53 RV5: RotaTeq 53 Tetanus Toxoid Vaccine TT: Tetanus Toxoid 57 Vaccine Storage and Handling Guide —————————————————————————————— -

COVID-19 Vaccineadmin VAERS
Reporting a Suspected Vaccine Adverse Event Indian Health Service, National Pharmacy & Therapeutics Committee CAPT Matthew Clark, MD, FAAP, FACP, Chair CAPT Ryan Schupbach, PharmD, BCPS, CACP, Vice Chair CAPT Christopher Lamer, PharmD, MHS, BCPS, CDE, Director of Pharmacovigilance October 2020 Potential New Vaccine for COVID-19 • Operation Warp Speed (OWS) • Collaboration between the Federal Government and biopharmaceutical companies to develop medications, diagnostic tests, and vaccines. • Shortened timelines but safety and efficacy are the primary focus. • Vaccines must be at least 50% effective for FDA EUA or approval. • Vaccines must be safe, and benefits of immunization must outweigh any risks of adverse events. • Continued need for influenza immunization • Continued need for scheduled immunizations https://www.hhs.gov/coronavirus/explaining-operation-warp-speed/index.html 2 Vaccine Safety: Common Adverse Events Vaccines are considered to be safe and effective with most common adverse events being mild and are signs that the body is developing immunity: • Pain, swelling, or redness where the shot was given • Mild fever • Chills • Feeling tired • Headache • Muscle and joint aches https://www.vaccines.gov/basics/safety/side_effects 3 Vaccine Safety: Serious Adverse Events More serious side effects are rare but can occur. Some examples are: • Anaphylaxis (0.65 cases/1 million vaccinations) • Thrombocytopenia from Rubella vaccine (1 case/40,000 vaccinations) • Orchitis from Mumps vaccine (0.3 cases/1 million vaccinations) • Intussusception from Rotavirus vaccine (1 case/100,000 vaccinations) • Guillain-Barre from flu vaccine (1 case/1.25 million vaccinations; association is stronger with flu infection than the vaccine) Spencer JP, Trondsen Pawlowski RH, Thomas S. Vaccine Adverse Events: Separating Myth from Reality. -

1 Title: Interim Report of a Phase 2 Randomized Trial of a Plant
medRxiv preprint doi: https://doi.org/10.1101/2021.05.14.21257248; this version posted May 17, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. 1 Title: Interim Report of a Phase 2 Randomized Trial of a Plant-Produced Virus-Like Particle 2 Vaccine for Covid-19 in Healthy Adults Aged 18-64 and Older Adults Aged 65 and Older 3 Authors: Philipe Gobeil1, Stéphane Pillet1, Annie Séguin1, Iohann Boulay1, Asif Mahmood1, 4 Donald C Vinh 2, Nathalie Charland1, Philippe Boutet3, François Roman3, Robbert Van Der 5 Most4, Maria de los Angeles Ceregido Perez3, Brian J Ward1,2†, Nathalie Landry1† 6 Affiliations: 1 Medicago Inc., 1020 route de l’Église office 600, Québec, QC, Canada, G1V 7 3V9; 2 Research Institute of the McGill University Health Centre, 1001 Decarie St, Montreal, 8 QC H4A 3J1; 3 GlaxoSmithKline Biologicals SA (Vaccines), Avenue Fleming 20, 1300 Wavre, 9 Belgium; 4 GlaxoSmithKline Biologicals SA (Vaccines), rue de l’Institut 89, 1330 Rixensart, 10 Belgium; † These individuals are equally credited as senior authors. 11 * Corresponding author: Nathalie Landry, 1020 Route de l’Église, Bureau 600, Québec, Qc, 12 Canada, G1V 3V9; Tel. 418 658 9393; Fax. 418 658 6699; [email protected] 13 Abstract 14 The rapid spread of SARS-CoV-2 globally continues to impact humanity on a global scale with 15 rising morbidity and mortality. Despite the development of multiple effective vaccines, new 16 vaccines continue to be required to supply ongoing demand.