Advisory Opinion on the Public Readiness and Emergency Preparedness Act and the March 10, 2020 Declaration Under the Act April 17, 2020, As Modified on May 19, 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
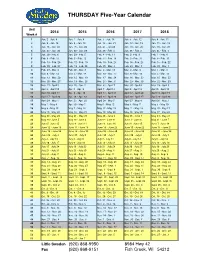
Thursday Calendar 20142018
THURSDAY Five-Year Calendar Unit 2014 20152016 2017 2018 Week # 1 Jan. 2 - Jan. 9 Jan. 1 - Jan. 8 Jan. 7 - Jan. 14 Jan. 5 - Jan. 12 Jan. 4 - Jan. 11 2 Jan. 9 - Jan. 16 Jan. 8 - Jan. 15 Jan. 14 - Jan. 21 Jan. 12 - Jan. 19 Jan. 11 - Jan. 18 3 Jan. 16 - Jan. 23 Jan. 15 - Jan. 22 Jan. 21 - Jan 28 Jan. 19 - Jan. 26 Jan. 18 - Jan. 25 4 Jan. 23 - Jan. 30 Jan. 22 - Jan. 29 Jan. 28 - Feb. 4 Jan. 26 - Feb. 2 Jan. 25 - Feb. 1 5 Jan. 30 - Feb. 6 Jan. 29 - Feb. 5 Feb. 4 - Feb. 11 Feb. 2- Feb. 9 Feb. 1 - Feb. 8 6 Feb. 6 - Feb. 13 Feb. 5 - Feb. 12 Feb. 11 - Feb. 18 Feb. 9 - Feb. 16 Feb. 8 - Feb. 15 7 Feb. 13 - Feb. 20 Feb. 12 - Feb. 19 Feb. 18 - Feb. 25 Feb. 16 - Feb. 23 Feb. 15 - Feb. 22 8 Feb. 20 - Feb. 27 Feb. 19 - Feb. 26 Feb. 25 - Mar. 3 Feb. 23 - Mar. 2 Feb. 22 - Mar. 1 9 Feb. 27 - Mar. 6 Feb. 26 - Mar. 5 Mar. 3 - Mar. 10 Mar. 2 - Mar. 9 Mar. 1 - Mar. 8 10 Mar. 6 - Mar. 13 Mar. 5 - Mar. 12 Mar. 10 - Mar. 17 Mar. 9 - Mar. 16 Mar. 5 - Mar. 15 11 Mar. 13 - Mar. 20 Mar. 12 - Mar. 19 Mar. 17 - Mar. 24 Mar. 16 - Mar. 23 Mar. 15 - Mar. 22 12 Mar. 20 - Mar. 27 Mar. 19 - Mar. 26 Mar. 24 - Mar. 31 Mar. 23 - Mar. 30 Mar. 22 - Mar. -

JUDICIAL ETHICS ADVISORY OPINION SUMMARIES May-June
JUDICIAL ETHICS ADVISORY OPINION SUMMARIES May-June 2019 Alaska Advisory Opinion 2019-1 http://www.acjc.alaska.gov/advopinions.html#2019-01 Disqualification: Former public defender A former public defender is disqualified from matters involving former clients for 2 years after becoming a judge unless the disqualification is waived but is not disqualified from cases against the state or a municipality or from cases in which the public defender’s office appears. Arizona Advisory Opinion 2019-1 http://www.azcourts.gov/Portals/137/ethics_opinions/2019/ethics%20opinion%2019-01.pdf Unsolicited donation for problem-solving court incentives A court may accept unsolicited monetary donations from a charitable organization to purchase incentives such as gift cards and bus passes for participants in problem-solving courts. Florida Advisory Opinion 2019-17 http://www.jud6.org/LegalCommunity/LegalPractice/opinions/jeacopinions/2019/2019- 17.html Disqualification: Parents’ former law firm, parents’ tenant A judge is not required to disqualify from cases involving her lawyer-parent’s former law firm. A judge is not automatically recused from cases involving a law firm that leases a building from her parent if her parent’s interest can be classified as de minimis. Florida Advisory Opinion 2019-18 http://www.jud6.org/LegalCommunity/LegalPractice/opinions/jeacopinions/2019/2019- 18.html Writing book A judge may write a book about family law courts and the mental health issues sometimes associated with them, specifically, the “warning signs” that judges and litigants should be concerned about, and may actively promote the book as long as he does not use the prestige of office to promote the book and the judge, his judicial assistant, and members of his family do not sell the book to any member of the Bar. -

March 10, 2021 COVID-19 Status Report Oak Park Village Board of Trustees
March 10, 2021 COVID-19 Status Report Oak Park Village Board of Trustees To: Village President and Village Board of Trustees Fr: Cara Pavlicek, Village Manager The memo is a weekly status report presented Wednesdays and provides a brief summary of information regarding Village of Oak Park operational activities in response to COVID-19, with an increasing focus on vaccination administration and allocation. In most cases, formal public guidance or employee guidance has been publicly disseminated via the Village website or Village social media channels. New Public Health Guidance Today, the Village of Oak Park Department of Public Health received notification of the following new COVID-19 cases: Date # of Cases Age of Cases March 4th 2 <20 to 30s March 5th 5 <20 to 70s March 6th 6 <20 to 70s March 7th 5 <20 to 40s March 8th 7 30s to 60s March 9th 8 <20 to 60s March 10th 2 30s to 80s Excluding individuals for which street addresses are not yet determined, the State of Illinois reports a total of two thousand seven hundred and ninety-six (2796) Oak Park COVID-19 cases. It is important to note that as patient tracking and case follow-up occurs, individuals listing the number of Oak Park cases reported may change depending on residency confirmation. The Oak Park Department of Public Health is notified of positive tests as established by state and local public health protocol. Because of privacy laws, no additional information can be released about the individuals. It is noted, that privacy precludes location information regarding individuals tested to anyone other than Public Health Officials and First Responders. -
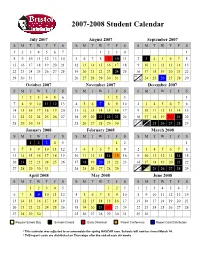
Approved Student Calendar
2007-2008 Student Calendar July 2007 August 2007 September 2007 SMTWT F S SMTWT F S SMTWT F S 1234567 1234 1 8910111213145678910 11 2 3 45678 15 16 17 18 19 20 21 12 13 14 15 16 17 18 9 10 11 12 13 14 15 22 23 24 25 26 27 28 19 20 21 22 23 24 25 16 17 18 19 20 21 22 23 29 30 31 26 27 28 29 30 31 30 24 25 26 27 28 29 October 2007 November 2007 December 2007 SMTWT F S SMTWT F S SMTWT F S 123456 123 1 7891011 12 134567 89102345678 14 15 16 17 18 19 20 11 12 13 14 15 16 17 9 10 11 12 13 14 15 21 22 23 24 25 26 27 18 19 20 21 22 23 24 16 17 18 19 20 21 22 23 24 28 29 30 31 25 26 27 28 29 30 30 31 25 26 27 28 29 January 2008 February 2008 March 2008 SMTWT F S SMTWT F S SMTWT F S 12345 12 1 67891011123456789 2345678 13 14 15 16 17 18 19 10 11 12 13 14 1516 9 1011121314 15 20 21 22 23 24 25 26 17 18 19 20 21 22 23 16 17 18 19 20 21 22 23 24 27 28 29 30 31 24 25 26 27 28 29 30 31 25 26 27 28 29 April 2008 May 2008 June 2008 SMTWT F S SMTWT F S SMTWT F S 12345 123 1234567 6789 10111245678910891011121314 13 14 15 16 17 18 19 11 12 13 14 15 16 17 15 16 17 18 19 20 21 20 21 22 23 24 25 26 18 19 20 21 22 23 24 22 23 24 25 26 27 28 27 28 29 30 25 26 27 28 29 30 31 29 30 Regular School Day Schools Closed Early Dismissal Parent Conference Report Card Distribution * This calendar was adjusted to accommodate the spring NASCAR race. -

2021-2022 Custom & Standard Information Due Dates
2021-2022 CUSTOM & STANDARD INFORMATION DUE DATES Desired Cover All Desired Cover All Delivery Date Info. Due Text Due Delivery Date Info. Due Text Due May 31 No Deliveries No Deliveries July 19 April 12 May 10 June 1 February 23 March 23 July 20 April 13 May 11 June 2 February 24 March 24 July 21 April 14 May 12 June 3 February 25 March 25 July 22 April 15 May 13 June 4 February 26 March 26 July 23 April 16 May 14 June 7 March 1 March 29 July 26 April 19 May 17 June 8 March 2 March 30 July 27 April 20 May 18 June 9 March 3 March 31 July 28 April 21 May 19 June 10 March 4 April 1 July 29 April 22 May 20 June 11 March 5 April 2 July 30 April 23 May 21 June 14 March 8 April 5 August 2 April 26 May 24 June 15 March 9 April 6 August 3 April 27 May 25 June 16 March 10 April 7 August 4 April 28 May 26 June 17 March 11 April 8 August 5 April 29 May 27 June 18 March 12 April 9 August 6 April 30 May 28 June 21 March 15 April 12 August 9 May 3 May 28 June 22 March 16 April 13 August 10 May 4 June 1 June 23 March 17 April 14 August 11 May 5 June 2 June 24 March 18 April 15 August 12 May 6 June 3 June 25 March 19 April 16 August 13 May 7 June 4 June 28 March 22 April 19 August 16 May 10 June 7 June 29 March 23 April 20 August 17 May 11 June 8 June 30 March 24 April 21 August 18 May 12 June 9 July 1 March 25 April 22 August 19 May 13 June 10 July 2 March 26 April 23 August 20 May 14 June 11 July 5 March 29 April 26 August 23 May 17 June 14 July 6 March 30 April 27 August 24 May 18 June 15 July 7 March 31 April 28 August 25 May 19 June 16 July 8 April 1 April 29 August 26 May 20 June 17 July 9 April 2 April 30 August 27 May 21 June 18 July 12 April 5 May 3 August 30 May 24 June 21 July 13 April 6 May 4 August 31 May 25 June 22 July 14 April 7 May 5 September 1 May 26 June 23 July 15 April 8 May 6 September 2 May 27 June 24 July 16 April 9 May 7 September 3 May 28 June 25. -

Published Ethics Advisory Opinions (Guide, Vol. 2B, Ch
Guide to Judiciary Policy Vol. 2: Ethics and Judicial Conduct Pt. B: Ethics Advisory Opinions Ch. 2: Published Advisory Opinions § 210 Index § 220 Committee on Codes of Conduct Advisory Opinions No. 2: Service on Governing Boards of Nonprofit Organizations No. 3: Participation in a Seminar of General Character No. 7: Service as Faculty Member of the National College of State Trial Judges No. 9: Testifying as a Character Witness No. 11: Disqualification Where Long-Time Friend or Friend’s Law Firm Is Counsel No. 17: Acceptance of Hospitality and Travel Expense Reimbursements From Lawyers No. 19: Membership in a Political Club No. 20: Disqualification Based on Stockholdings by Household Family Member No. 24: Financial Settlement and Disqualification on Resignation From Law Firm No. 26: Disqualification Based on Holding Insurance Policy from Company that is a Party No. 27: Disqualification Based on Spouse’s Interest as Beneficiary of a Trust from which Defendant Leases Property No. 28: Service as Officer or Trustee of Hospital or Hospital Association No. 29: Service as President or Director of a Corporation Operating a Cooperative Apartment or Condominium No. 32: Limited Solicitation of Funds for the Boy Scouts of America No. 33: Service as a Co-trustee of a Pension Trust No. 34: Service as Officer or on Governing Board of Bar Association No. 35: Solicitation of Funds for Nonprofit Organizations, Including Listing of Judges on Solicitation Materials No. 36: Commenting on Legal Issues Arising before the Governing Board of a Private College or University No. 37: Service as Officer or Trustee of a Professional Organization Receiving Governmental or Private Grants or Operating Funds No. -

March 10, 2021
March 10, 2021 Meeting Minutes Senators Present: Angela Haynie, Angela Jackson, Ashley Isom, Audreka Peten, Carlos Herrera, Deloise Mowdy, Dierre Littleton, Elizabeth Gayfield, Garth Clayborn, Henry Smiley, Hunter Roberts, Jason Cole, Jennifer Day, Julia Robison, Kristin Jetts, Kyana Smith, Maree Herring, Michael Hopper, Melanie Watson, Nadia Eslinger, Natalie Shock, Paul Dielmann, Richard Hammond, Shad Foley, Shelby Fiegel, Steven Shook, Susan Peterson, Tomas McDaniel, Senators Absent: Gunnar Bartlett, Jason Davis, Kimberly Klotz, Lynetta Morris, Taylor Ingram, I. Call to Order A. President Shook called the Staff Senate meeting to order at 10:01 a.m. on Zoom. II. Approval of February 24, 2021 Meeting Minutes A. The minutes from the February 24, 2021 meeting were reviewed via email and approved electronically. II. Guest Speaker: Dr. Pastor A. Dr. Pastor shared his screen and went over various models of COVID trend predictions as well as the actual trends. He then opened the room for any questions. 1. Senator Fiegel: What changes do you imagine will take place on campus, and how will that transition work? Answer: This transition will not work like a light switch. This will be a slow process and we are still going to continue to ask everyone to wear masks through the end of the semester. 2. Senator Smiley: Do you know how many people in the UCA community have been vaccinated? Answer: We have had about 1,700 people who have been vaccinated just through Conway Regional. This number does not include anyone who was vaccinated in any other place other than Conway Regional. Please let Amy Whitehead know if you have been vaccinated elsewhere. -

2021 7 Day Working Days Calendar
2021 7 Day Working Days Calendar The Working Day Calendar is used to compute the estimated completion date of a contract. To use the calendar, find the start date of the contract, add the working days to the number of the calendar date (a number from 1 to 1000), and subtract 1, find that calculated number in the calendar and that will be the completion date of the contract Date Number of the Calendar Date Friday, January 1, 2021 133 Saturday, January 2, 2021 134 Sunday, January 3, 2021 135 Monday, January 4, 2021 136 Tuesday, January 5, 2021 137 Wednesday, January 6, 2021 138 Thursday, January 7, 2021 139 Friday, January 8, 2021 140 Saturday, January 9, 2021 141 Sunday, January 10, 2021 142 Monday, January 11, 2021 143 Tuesday, January 12, 2021 144 Wednesday, January 13, 2021 145 Thursday, January 14, 2021 146 Friday, January 15, 2021 147 Saturday, January 16, 2021 148 Sunday, January 17, 2021 149 Monday, January 18, 2021 150 Tuesday, January 19, 2021 151 Wednesday, January 20, 2021 152 Thursday, January 21, 2021 153 Friday, January 22, 2021 154 Saturday, January 23, 2021 155 Sunday, January 24, 2021 156 Monday, January 25, 2021 157 Tuesday, January 26, 2021 158 Wednesday, January 27, 2021 159 Thursday, January 28, 2021 160 Friday, January 29, 2021 161 Saturday, January 30, 2021 162 Sunday, January 31, 2021 163 Monday, February 1, 2021 164 Tuesday, February 2, 2021 165 Wednesday, February 3, 2021 166 Thursday, February 4, 2021 167 Date Number of the Calendar Date Friday, February 5, 2021 168 Saturday, February 6, 2021 169 Sunday, February -
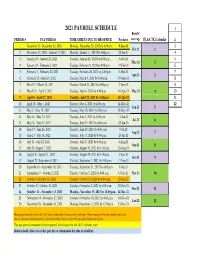
Payroll Calendar 2021
2021 PAYROLL SCHEDULE 1 Benefit PERIOD # PAY PERIOD TIME SHEETS DUE TO HR OFFICE Paydates coverage FLSA 7K Calendar 2 1 December 13- December 26, 2020 Monday, December 28, 2020 by 4:00 p.m. 8-Jan-21 3 Feb-21 1 2 December 27, 2020 - Janurary 9, 2021 Monday, January 11, 2021 by 4:00 p.m. 22-Jan-21 4 3 January 10 - January 23, 2021 Tuesday, January 26, 2021 by 4:00 p.m. 5-Feb-21 5 Mar-21 2 4 January 24 - February 6, 2021 Tuesday, February 9, 2021 by 4:00 p.m. 19-Feb-21 6 5 February 7 - February 20, 2021 Tuesday, February 26, 2021 by 4:00 p.m. 5-Mar-21 7 Apr-21 3 6 February 21 - March 6, 2021 Tuesday, March 9, 2021 by 4:00 p.m. 19-Mar-21 8 7 March 7 - March 20, 2021 Tuesday, March 23, 2021 by 4:00 p.m. 2-Apr-21 9 8 March 21 - April 3, 2021 Tuesday, April 6, 2021 by 4:00 p.m. 16-Apr-21 May-21 4 10 9 April 4 - April 17, 2021 Tuesday, April 20, 2021 by 4:00 p.m. 30-Apr-21 11 10 April 18 - May 1, 2021 Tuesday, May 4, 2021 by 4:00 p.m. 14-May-21 12 Jun-21 5 11 May 2 - May 15, 2021 Tuesday, May 18, 2021 by 4:00 p.m. 28-May-21 12 May 16 - May 29, 2021 Tuesday, June 1, 2021 by 4:00 p.m. 11-Jun-21 Jul-21 6 13 May 30 - June 12, 2021 Tuesday, June 15, 2021 by 4:00 p.m. -
![Advisory Opinion of 25 February 2019 [Amended 4 March 2019 by Request]](https://docslib.b-cdn.net/cover/6294/advisory-opinion-of-25-february-2019-amended-4-march-2019-by-request-826294.webp)
Advisory Opinion of 25 February 2019 [Amended 4 March 2019 by Request]
25 FÉVRIER 2019 AVIS CONSULTATIF EFFETS JURIDIQUES DE LA SÉPARATION DE L’ARCHIPEL DES CHAGOS DE MAURICE EN 1965 ___________ LEGAL CONSEQUENCES OF THE SEPARATION OF THE CHAGOS ARCHIPELAGO FROM MAURITIUS IN 1965 25 FEBRUARY 2019 ADVISORY OPINION TABLE OF CONTENTS Paragraphs CHRONOLOGY OF THE PROCEDURE 1-24 I. EVENTS LEADING TO THE ADOPTION OF THE REQUEST FOR THE ADVISORY OPINION 25-53 II. JURISDICTION AND DISCRETION 54-91 A. Jurisdiction 55-62 B. Discretion 63-68 1. Whether advisory proceedings are suitable for determination of complex and disputed factual issues 69-74 2. Whether the Court’s response would assist the General Assembly in the performance of its functions 75-78 3. Whether it would be appropriate for the Court to re-examine a question allegedly settled by the Arbitral Tribunal constituted under UNCLOS Annex VII in the Arbitration regarding the Chagos Marine Protected Area 79-82 4. Whether the questions asked relate to a pending dispute between two States, which have not consented to its settlement by the Court 83-91 III. THE FACTUAL CONTEXT OF THE SEPARATION OF THE CHAGOS ARCHIPELAGO FROM MAURITIUS 92-131 A. The discussions between the United Kingdom and the United States with respect to the Chagos Archipelago 94-97 B. The discussions between the Government of the United Kingdom and the representatives of the colony of Mauritius with respect to the Chagos Archipelago 98-112 C. The situation of the Chagossians 113-131 IV. THE QUESTIONS PUT TO THE COURT BY THE GENERAL ASSEMBLY 132-182 A. Whether the process of decolonization of Mauritius was lawfully completed having regard to international law (Question (a)) 139-174 1. -

Flex Dates.Xlsx
1st Day 1st Day of Your Desired Stay you may Call January 3, 2021 ↔ November 4, 2020 January 4, 2021 ↔ November 5, 2020 January 5, 2021 ↔ November 6, 2020 January 6, 2021 ↔ November 7, 2020 January 7, 2021 ↔ November 8, 2020 January 8, 2021 ↔ November 9, 2020 January 9, 2021 ↔ November 10, 2020 January 10, 2021 ↔ November 11, 2020 January 11, 2021 ↔ November 12, 2020 January 12, 2021 ↔ November 13, 2020 January 13, 2021 ↔ November 14, 2020 January 14, 2021 ↔ November 15, 2020 January 15, 2021 ↔ November 16, 2020 January 16, 2021 ↔ November 17, 2020 January 17, 2021 ↔ November 18, 2020 January 18, 2021 ↔ November 19, 2020 January 19, 2021 ↔ November 20, 2020 January 20, 2021 ↔ November 21, 2020 January 21, 2021 ↔ November 22, 2020 January 22, 2021 ↔ November 23, 2020 January 23, 2021 ↔ November 24, 2020 January 24, 2021 ↔ November 25, 2020 January 25, 2021 ↔ November 26, 2020 January 26, 2021 ↔ November 27, 2020 January 27, 2021 ↔ November 28, 2020 January 28, 2021 ↔ November 29, 2020 January 29, 2021 ↔ November 30, 2020 January 30, 2021 ↔ December 1, 2020 January 31, 2021 ↔ December 2, 2020 February 1, 2021 ↔ December 3, 2020 February 2, 2021 ↔ December 4, 2020 1st Day 1st Day of Your Desired Stay you may Call February 3, 2021 ↔ December 5, 2020 February 4, 2021 ↔ December 6, 2020 February 5, 2021 ↔ December 7, 2020 February 6, 2021 ↔ December 8, 2020 February 7, 2021 ↔ December 9, 2020 February 8, 2021 ↔ December 10, 2020 February 9, 2021 ↔ December 11, 2020 February 10, 2021 ↔ December 12, 2020 February 11, 2021 ↔ December 13, 2020 -

Advisory Opinion 2019-12
OPINION 2019-12 Issued October 4, 2019 Appointment of a Magistrate as an Eldercare Coordinator SYLLABUS: A probate court magistrate may not be appointed as an eldercare coordinator in addition to his or her duties as a judicial officer. The dual appointment of a probate court magistrate as an eldercare coordinator interferes with the official duties of the position of magistrate and leads to frequent disqualification. The dual appointment of a probate court magistrate as an eldercare coordinator raises questions as to the magistrate’s impartiality. This nonbinding advisory opinion is issued by the Ohio Board of Professional Conduct in response to a prospective or hypothetical question regarding the application of ethics rules applicable to Ohio judges and lawyers. The Ohio Board of Professional Conduct is solely responsible for the content of this advisory opinion, and the advice contained in this opinion does not reflect and should not be construed as reflecting the opinion of the Supreme Court of Ohio. Questions regarding this advisory opinion should be directed to the staff of the Ohio Board of Professional Conduct. 65 SOUTH FRONT STREET, 5TH FLOOR, COLUMBUS, OH 43215-3431 Telephone: 614.387.9370 Fax: 614.387.9379 www.bpc.ohio.gov HON. JOHN W. WISE RICHARD A. DOVE CHAIR DIRECTOR PATRICIA A. WISE D. ALLAN ASBURY VICE- CHAIR SENIOR COUNSEL KRISTI R. MCANAUL COUNSEL OPINION 2019-12 Issued October 4, 2019 Appointment of a Magistrate as an Eldercare Coordinator SYLLABUS: A probate court magistrate may not be appointed as an eldercare coordinator in addition to his or her duties as a judicial officer.