Tepzz¥ 4¥5 4A T
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Therapeutic Class Overview Irritable Bowel Syndrome Agents
Therapeutic Class Overview Irritable Bowel Syndrome Agents Therapeutic Class Overview/Summary: This review will focus on agents used for the treatment of Irritable Bowel Syndrome (IBS).1-5 IBS is a gastrointestinal syndrome characterized primarily by non-specific chronic abdominal pain, usually described as a cramp-like sensation, and abnormal bowel habits, either constipation or diarrhea, in which there is no organic cause. Other common gastrointestinal symptoms may include gastroesophageal reflux, dysphagia, early satiety, intermittent dyspepsia and nausea. Patients may also experience a wide range of non-gastrointestinal symptoms. Some notable examples include sexual dysfunction, dysmenorrhea, dyspareunia, increased urinary frequency/urgency and fibromyalgia-like symptoms.6 IBS is defined by one of four subtypes. IBS with constipation (IBS-C) is the presence of hard or lumpy stools with ≥25% of bowel movements and loose or watery stools with <25% of bowel movements. When IBS is associated with diarrhea (IBS-D) loose or watery stools are present with ≥25% of bowel movements and hard or lumpy stools are present with <25% of bowel movements. Mixed IBS (IBS-M) is defined as the presence of hard or lumpy stools with ≥25% and loose or water stools with ≥25% of bowel movements. Final subtype, or unsubtyped, is all other cases of IBS that do not fall into the other classes. Pharmacological therapy for IBS depends on subtype.7 While several over-the-counter or off-label prescription agents are used for the treatment of IBS, there are currently only two agents approved by the Food and Drug Administration (FDA) for the treatment of IBS-C and three agents approved by the FDA for IBS-D. -

Prophylactic Antiemetic Therapy with Ondansetron, Granisetron and Metoclopramide in Patients Undergoing Laparoscopic Cholecystectomy Under GA
JK SCIENCE ORIGINAL ARTICLE Prophylactic Antiemetic Therapy with Ondansetron, Granisetron and Metoclopramide in Patients Undergoing Laparoscopic Cholecystectomy Under GA Vishal Gupta, Renu Wakhloo, Anjali Mehta, Satya Dev Gupta Abstract The aim of the present study was to compare the antiemetic effect of intravenous Granisetron, Ondansetron & Metoclopramide in a randomized blinded study for prophylaxis of post operative nausea and vomiting (PONV) in patients undergoing laparoscopic cholecystectomy under general anaesthesia. 60 patients (ASA I & II) undergoing laparoscopic cholecystectomy under general anaesthesia were randomly allocated into three equal groups (n=20). Emetic episodes in first 24 hours were recorded and compared in different study groups. Results were analyzed. Minimal emetic episodes were observed in early post-operative period (1-12hrs) in patients who had received intravenous granisetron in comparison to ondansetron and metoclopramide. However, after 12 hours emesis free periods were statistically insignificant between group A and B while patients in group C had no antiemetic effect. Keywords Post Operative Nausea and Vomiting (PONV), Granisetron, Ondensetron, Metoclopramide Introduction The most common and distressing symptoms, which vascular anastomoses and increased intracranial follow anaesthesia and surgery, are pain and emesis. The pressure(4). The anaesthetic consequences are aspiration syndrome of nausea, retching and vomiting is known as pneumonitis and discomfort in recovery. For institutions 'sickness' and each part of it can be distinguished as a there is increased financial burden because of increased separate entity (1). PONV (post operative nausea and nursing care, delayed discharge from Phase I and II vomiting) has been characterized as big 'little problem(2) recovery units and unexpected admissions. Hence, and has been a common complication for both in patients prophylactic antiemetic therapy is needed for all these and out patients undergoing virtually all types of surgical patients. -

Comprehensive Pgx Report for 1 / 31 Examples of Different Levels of Evidence for Pgx Snps
Comprehensive PGx report for PERSONAL DETAILS Advanced Diagnostics Laboratory LLC CLIA:31D2149403 Phone: Fax: PATIENT DOB Address: 1030 North Kings Highway Suite 304 Cherry Hill, NJ 08034 GENDER FEMALE Website: http://advanceddiagnosticslaboratory.com/ SPECIMEN TYPE Oral Fluid LABORATORY INFORMATION ORDERING PHYSICIAN ACCESSION NUMBER 100344 FACILITY COLLECTION DATE 08/10/2020 RECEIVED DATE 08/14/2020 REPORT GENERATED 09/08/2020 LABORATORY DIRECTOR Dr. Jeanine Chiaffarano Current Patient Medication Clonidine (Catapres, Kapvay) The personalized pharmacogenomics profile of this patient reveals intermediate CYP2D6-mediated metabolism, extensive CYP1A2-mediated metabolism, and extensive CYP3A5-mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Losartan (Cozaar) The personalized pharmacogenomics profile of this patient reveals extensive CYP2C9-mediated metabolism, extensive CYP3A4-mediated metabolism, and extensive CYP3A5-mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Diltiazem (Cardizem, Tiazac) The personalized pharmacogenomics profile of this patient reveals extensive CYP3A4-mediated metabolism, intermediate CYP2C19-mediated metabolism, and extensive CYP3A5- mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Labetalol (Normodyne, Trandate) The personalized pharmacogenomics profile of this patient reveals intermediate CYP2D6-mediated metabolism, and intermediate CYP2C19-mediated metabolism. For further details, please find supporting evidence in this report or on websites such as www.pharmgkb.org or www.fda.gov. Mycophenolate mofetil (Myfortic, CellCept) The personalized pharmacogenomics profile of this patient reveals extensive CYP3A4-mediated metabolism, extensive CYP3A5-mediated metabolism, and extensive CYP2C8-mediated metabolism. -

ISSN 2320-5407 International Journal of Advanced Research (2014), Volume 2, Issue 12 , 53-58
ISSN 2320-5407 International Journal of Advanced Research (2014), Volume 2, Issue 12 , 53-58 Journal homepage: http://www.journalijar.com INTERNATIONAL JOURNAL OF ADVANCED RESEARCH RESEARCH ARTICLE Evaluation of the need of prophylactic antiemetic with injection Morphine in treating acute musculoskeletal pain in the Indian population. Dr. Amit Bhowmik, Dr. Indraneel Dasgupta, Dr.Sudeshna Barua, Dr. Ranjan Dutta Department of Emergency Medicine, Peerless Hospital and B. K. Roy Research Centre, Kolkata Manuscript Info Abstract Manuscript History: Objective: The objective of our study was to determine whether injection morphine cause nausea or vomiting in patients attending an Indian Received: 15 October 2014 Final Accepted: 26 November 2014 Emergency Department with acute musculoskeletal pain. Published Online: December 2014 Method: A prospective double-blinded trial was done on 236 patients with musculoskeletal trauma receiving intravenous morphine for analgesia. Key words: Children ≤ 18 years, patients who had been vomiting, raised ICP, or had Morphine, Nausea & Vomiting, already received prehospital analgesia or antiemetic, and those unable to give Prophylactic antiemetic consent were excluded from this study. Along with injection morphine – group one received Ramosetron, group two received Metoclopramide, group *Corresponding Author three received Promethazine and group four received placebo. Any vomiting Dr. Amit Bhowmik or nausea within 4 hours of receiving intravenous morphine was recorded. Result: The four groups were evenly matched for age groups, gender, comorbidities, trauma sites, morphine dose and antiemetic drug volumes. Overall, 12.4% of the patients experienced nausea (9.4% in the group receiving Ramosetron, 18.5% in the group receiving Metoclopramide, 14.3% in the group receiving Promethazine and 6.5% in the group receiving placebo) and 9.9% vomited (7.5% in the group receiving Ramosetron, 14.8% in the group receiving Metoclopramide, 10.2% in the group receiving Promethazine and 6.5% in the group receiving placebo). -

Twelve Cases of Drug-Induced Blepharospasm Improved Within 2 Months of Psychotropic Cessation
Drug, Healthcare and Patient Safety Dovepress open access to scientific and medical research Open Access Full Text Article CASE SERIES Twelve cases of drug-induced blepharospasm improved within 2 months of psychotropic cessation Yuko Emoto1 Background: To determine whether psychotropic cessation in patients with drug-induced Hirofumi Emoto2 blepharospasm improves motor symptoms. Eriko Oishi1 Methods: In patients with drug-induced blepharospasm, we withdrew part or all of their psy- Syunichi Hikita1 chotropic medication and assessed motor symptoms using the Jankovic rating scale (0 = none, Masato Wakakura1 1 = noticeable, 2 = mild, 3 = moderate, 4 = severe) at first presentation and after cessation. Results: Twelve patients (eleven women and one man, mean age 60.4 years) were enrolled. 1Division of Neuro-Ophthalmology, Psychotropics were administered before the onset of blepharospasm in all patients. The mean Inouye Eye Hospital, Tokyo; 2Department of Ophthalmology and duration of treatment with psychotropic medication was 47.3 (range 3–120) months. Jankovic Visual Science, Tokyo Medical and rating scale at initial presentation was 3 in eleven patients and 2 in one patient. After cessation, Dental University, Graduate School of Medicine, Tokyo, Japan blepharospasm started to improve in all cases within 2 months (average 3.9 weeks). While the effect of psychotropic cessation was variable, the symptoms eventually improved to more than 2 on the rating scale. Three of the twelve patients underwent a single botulinum neurotoxin injection and were withdrawn from therapy after cessation. Conclusion: Psychotropic drugs can cause blepharospasm in some cases. Clinicians should consider reducing psychotropic medication as far as possible in patients with blepharospasm taking these agents. -

5-HT3 Receptor Antagonists in Neurologic and Neuropsychiatric Disorders: the Iceberg Still Lies Beneath the Surface
1521-0081/71/3/383–412$35.00 https://doi.org/10.1124/pr.118.015487 PHARMACOLOGICAL REVIEWS Pharmacol Rev 71:383–412, July 2019 Copyright © 2019 by The Author(s) This is an open access article distributed under the CC BY-NC Attribution 4.0 International license. ASSOCIATE EDITOR: JEFFREY M. WITKIN 5-HT3 Receptor Antagonists in Neurologic and Neuropsychiatric Disorders: The Iceberg Still Lies beneath the Surface Gohar Fakhfouri,1 Reza Rahimian,1 Jonas Dyhrfjeld-Johnsen, Mohammad Reza Zirak, and Jean-Martin Beaulieu Department of Psychiatry and Neuroscience, Faculty of Medicine, CERVO Brain Research Centre, Laval University, Quebec, Quebec, Canada (G.F., R.R.); Sensorion SA, Montpellier, France (J.D.-J.); Department of Pharmacodynamics and Toxicology, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran (M.R.Z.); and Department of Pharmacology and Toxicology, University of Toronto, Toronto, Ontario, Canada (J.-M.B.) Abstract. ....................................................................................384 I. Introduction. ..............................................................................384 II. 5-HT3 Receptor Structure, Distribution, and Ligands.........................................384 A. 5-HT3 Receptor Agonists .................................................................385 B. 5-HT3 Receptor Antagonists. ............................................................385 Downloaded from 1. 5-HT3 Receptor Competitive Antagonists..............................................385 2. 5-HT3 Receptor -

Department of Health
DEPARTMENT OF HEALTH National Drug Policy - Pharmaceutical Management Unit 50 National Formulary Committee Philippine National Drug Formulary EssentialEssential MedicinesMedicines ListList Volume I, 7th Edition ( 2008 ) Published by: The National Formulary Committee National Drug Policy ‐ Pharmaceutical Management Unit 50 DEPARTMENT OF HEALTH Manila, Philippines All rights reserved 2008 The National Formulary Committee National Drug Policy‐Pharmaceutical Management Unit 50 (NDP‐PMU 50) Department of Health San Lazaro Cmpd., Rizal Ave., Sta. Cruz, Manila, Philippines 1003 ISBN 978‐971‐91620‐7‐0 Any part or the whole book may be reproduced or transmitted without any alteration, in any form or by any means, with permission from DOH provided it is not sold commercially. ii PHILIPPINE NATIONAL DRUG FORMULARY Volume I, 7th Edition 2 0 0 8 Francisco T. Duque III, MD, MSc Secretary of Health Alexander A. Padilla Undersecretary of Health, Office for External Affairs Robert Louie P. So, MD Program Manager, NDP-PMU 50 Dennis S. Quiambao, MD Proj. Mgmt. Operating Officer & Coordinator (PMOOC) NDP-PMU 50 NATIONAL FORMULARY COMMITTEE Estrella B. Paje-Villar, MD, DTM & H Chairperson Jose M. Acuin, MD, MSc Alma L. Jimenez, MD Alejandro C. Baroque II, MD Marieta B. de Luna, MD Bu C. Castro, MD Nelia P. Cortes-Maramba, MD Dina V. Diaz, MD Yolanda R. Robles, PhD Pharm Mario R. Festin, MD, MS, MHPEd Isidro C. Sia, MD BFAD Representative SECRETARIAT Luzviminda O. Marquez, RPh, RMT Mary Love C. Victoria, RPh Michael D. Junsay, RPh Ermalyn M. Magturo iii Republic of the Philippines DEPARTMENT OF HEALTH OFFICE OF THE SECRETARY 2/F Bldg. 1, San Lazaro Cmpd., Rizal Avenue, Sta. -

IBS Treatment
TREATMENTS OF IBS Douglas A. Drossman, MD Co-Director UNC Center for Functional GI & Motility Disorders INTRODUCTION In recent years, there has been increased interest by physicians and the pharmaceutical industry regarding newer treatments for IBS. Before discussing these new treatments, it is important to consider the overall management strategy in IBS. This is necessary because patients with IBS exhibit a wide spectrum of symptoms of varying frequencies and degrees of severity. There is no one ideal treatment for IBS, and the newer medications may work best for only a subset of patients having this disorder. Therefore, the clinician must first apply certain general management approaches and, following this, treatment choices will depend on the nature (i.e., predominant diarrhea, constipation, or bloating, etc.) and severity (mild, moderate, severe) of the symptoms. The symptoms of IBS may have any of several underlying causes. These can include: (a) abnormal motility (uncoordinated or excessive contractions that can lead to diarrhea, constipation, bloating) (b) visceral hypersensitivity (lower pain threshold of the nerves that can produce abdominal discomfort or pain) resulting from the abnormal motility, stress or infection (c) dysfunction of the brain's ability to regulate these visceral (intestinal) activities. Treatments will vary depending on which of these possibilities are occurring. In general, milder symptoms relate primarily to abnormal motility, often in response to food, activity or stress, and/or visceral hypersensitivity. They are commonly treated symptomatically with pharmacological agents directed at the gut. However, more severe symptoms often relate to dysfunction of the brain-gut regulatory system with associated psychosocial effects, and psychological or behavioral treatments and antidepressants are frequently helpful. -

ETIZOLAM Critical Review Report Agenda Item 4.13
ETIZOLAM Critical Review Report Agenda Item 4.13 Expert Committee on Drug Dependence Thirty-ninth Meeting Geneva, 6-10 November 2017 39th ECDD (2017) Agenda item 4.13 Etizolam Page 2 of 20 39th ECDD (2017) Agenda item 4.13 Etizolam Contents Acknowledgements.................................................................................................................................. 5 Summary...................................................................................................................................................... 6 1. Substance identification ....................................................................................................................... 7 A. International Nonproprietary Name (INN).......................................................................................................... 7 B. Chemical Abstract Service (CAS) Registry Number .......................................................................................... 7 C. Other Chemical Names ................................................................................................................................................... 7 D. Trade Names ....................................................................................................................................................................... 7 E. Street Names ....................................................................................................................................................................... 8 F. Physical Appearance -

Effects of Brotizolam, a New Thieno-Triazolo-Diazepine Derivative, on the Central Nervous System
In the present study, its actions on the central nervous system were investigated. Effects of Brotizolam, a New Thieno-Triazolo-Diazepine Derivative, on the Central Nervous System Kenjiro KIMISHIMA, Kyoko TANABE, Yukako KINOSHITA, Kooji TOKUYOSHI, Daisuke HOURI and Tatsuo KOBAYASHI Department of Pharmacology, Tottori University School of Medicine, Yonago 683, Japan Accepted August 24, 1984 Abstract-The effects of brotizolam, a new thieno-triazolo-diazepine derivative, on the central nervous system were analyzed in mice, rats and rabbits. Diazepam, estazolam and triazolam were used as control drugs. Brotizolam inhibited spon taneous motor activities; performances in the rotarod test, staircase test, and maximal electroshock seizure test; and pentetrazol or bemegride-induced convulsion. Moreover, catalepsy inducing action and potentiating effect on sleep elicited by pentobarbital or ethanol were observed. Following intraperitoneal or oral admin istration of brotizolam to rabbits with chronically implanted electrodes , the electro encephalographic profile in spontaneous EEG was characterized by slow waves with high amplitudes in the neocortex. The arousal responses by stimulation of the midbrain reticular formation and posterior hypothalamus were slightly inhibited, but the recruiting responses induced by stimulation of the diffuse thalamic projecting system were not inhibited, and seizure discharges induced by stimulation of the dorsal hippocampus were inhibited markedly. When motor activities and pente trazol-induced convulsions were observed as indices of tolerance for brotizolam, tolerance was not developed by repeated administration of brotizolam up to 14 days. These results suggested that brotizolam, a new thieno-triazolo-diazepine derivative, is judged to be a safer and stronger sleep inducer than diazepam and estazolam . Since the pioneering paper by Randall et et al. -
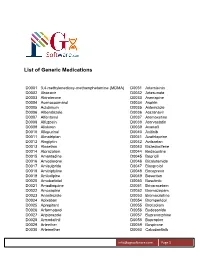
List of Generic Medications
List of Generic Medications D0001 3,4-methylenedioxy-methamphetamine (MDMA) D0031 Artemisinin D0002 Abacavir D0032 Artesunate D0003 Abiraterone D0033 Asenapine D0004 Acenocoumarol D0034 Aspirin D0005 Aclidinium D0035 Astemizole D0006 Albendazole D0036 Atazanavir D0007 Alfentanyl D0037 Atomoxetine D0008 Alfuzosin D0038 Atorvastatin D0009 Aliskiren D0039 Avanafil D0010 Allopurinol D0040 Axitinib D0011 Almotriptan D0041 Azathioprine D0012 Alogliptin D0042 Azilsartan D0013 Alosetron D0043 Bazedoxifene D0014 Alprazolam D0044 Bedaquiline D0015 Amantadine D0045 Bepridil D0016 Amiodarone D0046 Bicalutamide D0017 Amisulpride D0047 Bisoprolol D0018 Amitriptyline D0048 Boceprevir D0019 Amlodipine D0049 Bosentan D0020 Amobarbital D0050 Bosutinib D0021 Amodiaquine D0051 Brivaracetam D0022 Amoxapine D0052 Bromazepam D0023 Anastrozole D0053 Bromocriptine D0024 Apixaban D0054 Bromperidol D0025 Aprepitant D0055 Brotizolam D0026 Arformoterol D0056 Budesonide D0027 Aripiprazole D0057 Buprenorphine D0028 Armodafinil D0058 Bupropion D0029 Arteether D0059 Buspirone D0030 Artemether D0060 Cabozantinib [email protected] Page 1 List of Generic Medications D0061 Canagliflozin D0091 Clofarabine D0062 Cannabidiol (CBD) D0092 Clofibrate D0063 Cannabinol (CBN) D0093 Clomipramine D0064 Captopril D0094 Clonazepam D0065 Carbamazepine D0095 Clonidine D0066 Carisoprodol D0096 Clopidogrel D0067 Carmustine D0097 Clorazepate D0068 Carvedilol D0098 Clozapine D0069 Celecoxib D0099 Cocaine D0070 Ceritinib D0100 Codeine D0071 Cerivastatin D0101 Colchicine D0072 Cetirizine -

Efficacy of Eluxadoline in Irritable Bowel Syndrome with Diarrhea
This is a repository copy of Efficacy of Eluxadoline in Irritable Bowel Syndrome With Diarrhea. White Rose Research Online URL for this paper: http://eprints.whiterose.ac.uk/156693/ Version: Accepted Version Article: Black, CJ, Houghton, LA and Ford, AC orcid.org/0000-0001-6371-4359 (2020) Efficacy of Eluxadoline in Irritable Bowel Syndrome With Diarrhea. American Journal of Gastroenterology, 115 (3). pp. 483-484. ISSN 0002-9270 10.14309/ajg.0000000000000518 © The American College of Gastroenterology 2020. All Rights Reserved. This is an author produced version of correspondence published in The American Journal of Gastroenterology. Uploaded in accordance with the publisher's self-archiving policy. Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Black et al Page 1 of 11 TITLE PAGE Title: Relative Efficacy of Eluxadoline in Irritable Bowel Syndrome. Authors: Christopher J. Black1, 2, Lesley A. Houghton, 2, Alexander C. Ford1, 2. 1Leeds Gastroenterology Institute, St.