Ozone and Its Variability in Hong Kong Junyi Cai
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Supporting Statements
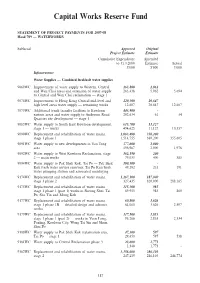
Capital Works Reserve Fund STATEMENT OF PROJECT PAYMENTS FOR 2007-08 Head 709 — WATERWORKS Subhead Approved Original Project Estimate Estimate Cumulative Expenditure Amended to 31.3.2008 Estimate Actual $’000 $’000 $’000 Infrastructure Water Supplies — Combined fresh/salt water supplies 9069WC Improvement of water supply to Western, Central 263,800 2,062 and Wan Chai areas and extension of water supply 263,438 5,962 5,694 to Central and Wan Chai reclamation — stage 1 9076WC Improvement to Hong Kong Central mid-level and 229,300 20,647 high level areas water supply — remaining works 12,607 20,647 12,607 9079WC Additional trunk transfer facilities to Kowloon 443,900 - eastern areas and water supply to Anderson Road 202,634 61 54 Quarries site development — stage 1 9085WC Water supply to South East Kowloon development, 615,700 11,127 stage 1 — works 494,625 11,127 10,357 9090WC Replacement and rehabilitation of water mains, 2,063,400 350,200 stage 1 phase 1 1,514,755 380,200 355,605 9091WC Water supply to new developments in Yau Tong 377,600 2,000 area 196,987 2,000 1,978 9092WC Water supply to West Kowloon Reclamation, stage 162,190 200 2 — main works 79,033 400 383 9094WC Water supply to Pak Shek Kok, Tai Po — Pak Shek 100,500 - Kok fresh water service reservoir, Tai Po Kau fresh 49,292 235 191 water pumping station and associated mainlaying 9174WC Replacement and rehabilitation of water mains, 1,267,100 187,000 stage 1 phase 2 327,435 320,000 288,185 9175WC Replacement and rehabilitation of water mains, 115,300 985 stage 1 phase 1 (part -

NA36 Yuen Long
Effective Date:From 00:01 on 20 June 2021 Bus Stop List for Long Win Route No. NA36 To Airport (Passenger Terminal Building/ To Yuen Long (Kam Sheung Road Station) Cathay Pacific City) No. Bus Stop Name (Note 1) Street No. Bus Stop Name (Note 1) Street Yuen Long Airport Unnamed Road 1) Kam Sheung Road Station 1) Cathay Pacific City (South Bound) Airport (Ground Transportation Centre) Bus 2) Ko Po Tsuen 2) Kam Tin Road Terminus 3) Ha Ko Po Tsuen (West Bound) HZMB Hong Kong Port 4) Au Tau 3) HZMB Hong Kong Port 5) Tung Shing Lei Tuen Mun Tuen Mun Chek Lap Kok Tunnel Interchange 6) Yeung Uk Tsuen 4) (T3) (Yuen Long Bound) Tuen Mun Town Plaza Tuen Hi Road 7) YOHO MALL I 5) [near (North Bound) Tuen Mun Central] Tuen Mun Road 8) Yau San Street 6) Hugn Kiu Castle Peak Road (North Bound) 9) Tai Tong Road (West Bound) Yuen Long Wang Tat Road 10) Hong Lok Road 7) Fung Chi Tsuen (East Bound) Yuen Long Police 11) 8) Yuen Long Plaza Station Castle Peak Road 12) Shui Pin Tsuen 9) Tung Lok Street (East Bound) 13) Yuen Long Park 10) Kuk Ting Street Long Yat Road 11) YOHO MALL II Tuen Mun (East Bound) Tuen Mun Road Long Yat Road 14) Hung Kiu 12) YOHO MALL I (South Bound) (South Bound) Waldorf Garden Tuen Fat Road Castle Peak Road 15) [near 13) Tung Shing Lei (South Bound) (East Bound) Tuen Mun Central] Tuen Mun Chek Lap Kok Tunnel Interchange 16) 14) Au Tau (A1) (Airport bound) Kam Tin Road 15) Ha Ko Po Tsuen HZMB Hong Kong Port (East Bound) HZMB Passenger Shun Fai Road 17) 16) Ko Po Tsuen Clearance Building (East Bound) Airport 17) Kam Sheung Road Station Cheong Hong Road 18) Terminal 1 (North Bound) Scenic Road 19) Cathay Pacific City (South Bound) Note 1: Passengers can click on the hyperlink for the above bus stop name to check for the location and the street view of the bus stops. -

List of Doctors / Clinics Enrolled in COVID-19 Vaccination Programme Under the Vaccination Subsidy Scheme
List of Doctors / Clinics Enrolled in COVID-19 Vaccination Programme under the Vaccination Subsidy Scheme Yuen Long District Name of Doctor Name of Clinic Address Enquiry Phone No. Podium M14, Shek Ping House, Long Ping Estate, YUEN LONG, NEW CHAK, CHI WAH Dr Chak Chi Wah's Clinic 24758988 TERRITORIES Shop G, 1/F, Phase 2, Kingswood Richly Plaza, Kingswood Villas, TIN SHUI CHAN, CHI WAI NIXON Grace Medical Clinic 31522170 WAI, NEW TERRITORIES CHAN, HUNG WAI Dr Chan Hung Wai Patrick Flat E15, Town Centre, Fairview Park, YUEN LONG, NEW TERRITORIES 24719580 PATRICK Shop NG16, G/F, T Town North, Tin Yuet Estate, 33 and 39 Tin Wah Road, Tin CHAN, KUNG YAT Dr Chan Kung Yat 22530543 Shui Wai, YUEN LONG, NEW TERRITORIES Shop 5, G/F, Chestwood Court, Kingswood Villas, 8 Tin Shui Road, TIN SHUI CHAN, LAM FUNG Heavenly Joy Family Clinic 26169898 WAI, NEW TERRITORIES Shop 7, G/F, Kwong Wah Plaza, 11 Tai Tong Road, YUEN LONG, NEW CHAN, NAI YIN Dr. Chan Nai Yin 24422120 TERRITORIES CHAN, TSE PUI Dr Chan Tse Pui G/F, Fu Shing Building, 8 Sai Ching Street, YUEN LONG, NEW TERRITORIES 24792716 Shop C2C & C3A, 1/F , Kingswood Richly Plaza, Kingswood Villas, TIN SHUI CHAN, TSZ KIN ANTHONY Tsz Kin Medical Centre 26170508 WAI, NEW TERRITORIES CHAN, WAI CHUNG Jockey Club Tin Shui Wai Community Health Unit 103, 1/F, Tin Ching Amenity & Community Building, Tin Ching Estate, Tin 31569000 VIRGINIA Centre Kwai Road, TIN SHUI WAI, NEW TERRITORIES Shop F1, 1/F, Phase 2, Kingswood Richly Plaza, Kingswood Villas, TIN SHUI CHEUNG, HON YUEN Town Health-PHC Medical Centre 21461119 WAI, NEW TERRITORIES 1 List of Doctors / Clinics Enrolled in COVID-19 Vaccination Programme under the Vaccination Subsidy Scheme Yuen Long District Name of Doctor Name of Clinic Address Enquiry Phone No. -

Hong Kong's Civil Disobedience Under China's Authoritarianism
Emory International Law Review Volume 35 Issue 1 2021 Hong Kong's Civil Disobedience Under China's Authoritarianism Shucheng Wang Follow this and additional works at: https://scholarlycommons.law.emory.edu/eilr Recommended Citation Shucheng Wang, Hong Kong's Civil Disobedience Under China's Authoritarianism, 35 Emory Int'l L. Rev. 21 (2021). Available at: https://scholarlycommons.law.emory.edu/eilr/vol35/iss1/2 This Article is brought to you for free and open access by the Journals at Emory Law Scholarly Commons. It has been accepted for inclusion in Emory International Law Review by an authorized editor of Emory Law Scholarly Commons. For more information, please contact [email protected]. WANG_2.9.21 2/10/2021 1:03 PM HONG KONG’S CIVIL DISOBEDIENCE UNDER CHINA’S AUTHORITARIANISM Shucheng Wang∗ ABSTRACT Acts of civil disobedience have significantly impacted Hong Kong’s liberal constitutional order, existing as it does under China’s authoritarian governance. Existing theories of civil disobedience have primarily paid attention to the situations of liberal democracies but find it difficult to explain the unique case of the semi-democracy of Hong Kong. Based on a descriptive analysis of the practice of civil disobedience in Hong Kong, taking the Occupy Central Movement (OCM) of 2014 and the Anti-Extradition Law Amendment Bill (Anti-ELAB) movement of 2019 as examples, this Article explores the extent to which and how civil disobedience can be justified in Hong Kong’s rule of law- based order under China’s authoritarian system, and further aims to develop a conditional theory of civil disobedience for Hong Kong that goes beyond traditional liberal accounts. -

Kingswood Villas (Maywood Court), Tin Shui
N.T.R.S. PSL No. 3658A Route No. NR906 SCHEDULE OF SERVICE New Territories Residents’ Service Route No. 906R I ROUTE KINGSWOOD VILLAS (MAYWOOD COURT), TIN SHUI WAI to KWUN TONG : via estate road of Maywood Court, Tin Lung Road, Tin Kwai Road, Tin Wah Road, Tin Shing Road, Tin Wu Road, estate road of Locwood Court, Tin Wu Road, Tin Shing Road, Long Tin Road, Tong Yan San Tsuen Interchange, Yuen Long Highway, Tsing Long Highway, Ting Kau Interchange, Ting Kau Bridge, North West Tsing Yi Interchange, Cheung Tsing Highway, Cheung Tsing Tunnel, Tsing Kwai Highway, unnamed access road, Ching Cheung Road, Lung Cheung Road, Lung Poon Street, Fung Tak Road, Hammer Hill Road, Lung Cheung Road, Kwun Tong Road, Hong Ning Road, Mut Wah Street, Hip Wo Street, Kwun Tong Road, Tsui Ping Road, roundabout and Tsui Ping Road. KWUN TONG to KINGSWOOD VILLAS (MAYWOOD COURT), TIN SHUI WAI : via Tsui Ping Road, Lei Yue Mun Road, Wai Fat Road, Cha Kwo Ling Road, Kwun Tong Road, Lung Cheung Road, Hammer Hill Road, Fung Tak Road, Po Kong Village Road, Lung Poon Street, Fung Tak Road, Po Kong Village Road, unnamed access road to Lung Cheung Road, Lung Cheung Road, Ching Cheung Road, unnamed access road, Tsing Kwai Highway, Cheung Tsing Tunnel, Cheung Tsing Highway, North West Tsing Yi Interchange, Ting Kau Bridge, Ting Kau Interchange, Tsing Long Highway, Yuen Long Highway, Tong Yan San Tsuen Interchange, Long Tin Road, Tin Tsz Road, Tin Cheung Road, Tin Shing Road, Tin Wu Road, estate road of Locwood Court, Tin Wu Road, Tin Shing Road , Tin Lung Road and estate road of Maywood Court. -

Palaeozoic Rocks of the San Tin Group Classification and Distribution
PalaeozoicRocks of the San Tin Group Classification and Distribution The sedimenmryrocks of this Palaeozoicbasin (the San Tin Group) occupy a northeasterly,curving, faulted, irregular belt at least 25 km long and up to a maximum of 4 km in width. This fault-bounded basinextends northwards into Shenzhenand Guangdong,and south throughTuen Mun. Bennett (1984c) outlined the basic structureof the areaas a narrow grabenbetween the CastlePeak and the Sung Kong granites,and noted the presenceof metasedimenmryrocks of the Repulse Bay Formation and the Lok Ma ChauFormation. The San Tin Group is divided into two formations; a lower, largely calcareousYuen Long Formation, and an upper, mostly arenaceous/argillaceousLok Ma ChauFormation (Langford et ai, 1989)(Figure5). Yuen Long Formation The Yuen Long Fonnation was named by Lee (1985) to distinguish the concealed marbles and limestonesof the Yuen Long area from the better known clastic rocks belonging to the establishedand exposed Lok Ma Chau Fonnation (Bennett, 1984b). The distinctive carbonate lithologies were originally recognised by Ha et al (1981), who suggested that they probably belonged to the CarboniferousPeriod. General supportfor a Carboniferousage was provided by the strike of the rocks which could be traced northeastwardsinto Shenzhen,where unpublished1:50 000 geologicalmapping of the Shenzhen,Special Economic Zone apparently showed similar lithologies classified as Lower Carboniferous(Visean) (Lai & Mui, 1985). The Yuen Long Fonnation is overlain by the Lok Ma Chau Fonnation. The boundary betweenthe two fonnations is in places gradationalbut in others sharp and probably unconfonnable.The presenceof beds of marble intercalatedwith the lowest metasiltstonesin someboreholes is interpretedby Langford et al (1989) to be a gradual passagefrom a dominantly calcareoussequence to one of largely clastic material. -

Project Profile for Yuen Long South Effluent Polishing Plant
THE GOVERNMENT OF THE HONG KONG SPECIAL ADMINISTRATIVE REGION DRAINAGE SERVICES DEPARTMENT Project Profile for Yuen Long South Effluent Polishing Plant January 2019 Yuen Long South Effluent Polishing Plant Project Profile CONTENTS 1. BASIC INFORMATION .............................................................................................................. 2 1.1 Project Title ...................................................................................................................................... 2 1.2 Purpose and Nature of the Project .................................................................................................... 2 1.3 History of Site .................................................................................................................................. 2 1.4 Name of the Project Proponent ........................................................................................................ 2 1.5 Location and Scale of the Project ..................................................................................................... 3 1.6 Type of Designated Project covered by the Project Profile ............................................................. 3 1.7 Name and Telephone Number of Contact Persons .......................................................................... 4 2. OUTLINE OF PLANNING AND IMPLEMENTATION PROGRAMME ............................ 5 2.1 Project Planning and Implementation ............................................................................................. -

Hong Kong's Old Villages
METUPLACES JFA FROM 2018/2 THE PAST LOST IN NEW TOWNS: DOI:METU 10.4305/METU.JFA.2017.2.5 JFA 2018/2 197 (35:2)HONG 197-220 KONG’S OLD VILLAGES PLACES FROM THE PAST LOST IN NEW TOWNS: HONG KONG’S OLD VILLAGES Terry Van DIJK*, Gerd WEITKAMP** Received: 24.02.2016; Final Text: 06.03.2017 INTRODUCTION Keywords: Heritage; new town; master plan; planning; urbanisation. Awareness of Hong Kong’s built heritage and its value is considered to have begun to increase around the time of the end of British rule. The change in Hong Kong’s sovereignty in 1997 prompted a search for its own identity, because while no longer under British rule, and not being nor becoming entirely Chinese, it was not immediately obvious what the emerging Hong Kong should put forward as its cultural identity. The question since that time has also become economically pertinent, as Hong Kong has developed into a major Asian tourist destination. As cultural tourism could be developed into one of the pillars of Hong Kong’s leisure economy, debate emerged on its identity and the built heritage it reflects. This article addresses the popular assumption that before 1997, heritage had been of little interest to Hong Kong’s governments, as articulated by Yung and Chan (2011), Henderson (2001) and Cheung (1999). This negligence was explained by the fact that Hong Kong’s population was growing exponentially through several waves of large-scale immigration, while being under an obviously temporary British government. This resulted in a heterogeneous population (Henderson, 2001) which had just migrated there and was more concerned about access to housing, employment and transportation than the history of the lands they were about to inhabit (Yung and Chan, 2011, 459). -

Guangdong-Hong Kong-Macao Pearl River Delta Regional Air Quality Monitoring Network
Guangdong-Hong Kong-Macao Pearl River Delta Regional Air Quality Monitoring Network April to June 2016 Statistical Summary of the Second Quarter Monitoring Results Report Number : PRDAIR-2016-2 Report Prepared by : Guangdong Provincial Environmental Monitoring Centre Environmental Protection Department, Hong Kong SARG Environmental Protection Bureau, Macao SARG Meteorological and Geophysical Bureau, Macao SARG Approved by : Quality Management Committee of Guangdong-Hong Kong-Macao Pearl River Delta Regional Air Quality Monitoring Network Security Classification : Unrestricted Contents Page 1. Foreword 3 2. Introduction to Guangdong-Hong Kong-Macao Pearl River Delta Regional Air Quality Monitoring Network 3 3. Operation of the Network 4 4. Statistical Analysis of Pollutant Concentrations 4 Annex A: Site Information of Monitoring Stations 21 Annex B: Measurement Methods of Air Pollutant Concentration 22 List of Tables Page Table 4.1a: The monthly maxima and mimima of hourly averages of SO2 5 Table 4.1b: The monthly maxima and mimima of daily averages of SO2 6 Table 4.1c: The monthly averages of SO2 7 Table 4.2a: The monthly maxima and mimima of hourly averages of NO2 8 Table 4.2b: The monthly maxima and mimima of daily averages of NO2 9 Table 4.2c: The monthly averages of NO2 10 Table 4.3a: The monthly maxima and mimima of hourly averages of O3 11 Table 4.3b: The monthly maxima and mimima of daily maximum 8-hour averages of O3 12 Table 4.3c: The monthly averages of O3 13 Table 4.4a: The monthly maxima and mimima of hourly averages of CO 14 Table 4.4b: The monthly maxima and mimima of daily averages of CO 15 Table 4.4c: The monthly averages of CO 16 Table 4.5a: The monthly maxima and mimima of daily averages of PM10 17 Table 4.5b: The monthly averages of PM10 18 Table 4.6a: The monthly maxima and mimima of daily averages of PM2.5 19 Table 4.6b: The monthly averages of PM2.5 20 List of Figures Page Figure 2.1: Spatial Distribution of Monitoring Stations in the Network 4 1. -

Yuen Long / Hung Shui Kiu / Tin Shui
Effective Date: From The First Departure on 30 August 2021 Suggested Choices Between Yuen Long / Tin Shui Wai And Airport / Hong Kong Port of Hong Kong‐Zhuhai‐Macao Bridge (“HZMB HKP”) Airport and Airport HZMB Cargo Terminal 1 Cathay HZMB HKP AsiaWorld‐ and Pacific HKP Expo Catering Yuen Long, City Tin Shui Wai Area ⇋ YOHO MALL, A36 A36 E36 / E36 / A36 Yuen Long Station E32 / E41 E36S E36S Castle Peak Road – A36 ⇋ E36 / E36 / Yuen Yuen Long (Yuen A36 E32 / E41 E36S E36S A36 Long Long Main Road) Long Ping Estate, A37 ⇋ A37 ⇋ A37 ⇋ A37 ⇋ Long Ping Station, S64/S64X/ E33/E33P/ A37 E32 / E41 Wang Fu Count S65 E36/E37 A33X/A36 Riva, A36 ⇋ E36 / E36P Ko Po A36 E32 / E41 A36 Au Tau, KMB 251A KMB 64K ⇋ E36; KMB 64K Kam Tin, Kam Sheung Road E36P or E36P Pat ⇋A43/A43P ⇋ A36 Kam Tin Road Heung KMB 251B KMB 54 / 77K ⇋ E36; KMB 54/ (Sheung Tsuen to E36P or E36P Kam Tin) ⇋A43/A43P 77K ⇋ A36 A37 (Rapid)/ A37 (Rapid)/ Hung Shui Kiu, A37 (Rapid)/ A37(Rapid)/ Hung A37(Rapid)/ A34 ⇋ A34 ⇋ Hung Fuk Estate / A34 ⇋ Shui Kiu S64/S64X/ E33 / E33P A34 ⇋ Shek Po Tsuen A34 E32 / E41 S65 / E36 / E37 A33X/A36 Tin Shui Wai Town Centre, Harbour Plaza Resort City, A37 ⇋ Kenswood Court, A37 ⇋ E37 E37 Lynwood Court, A37 E32 / E41 A33X / Tin Shui Tin Ching, Tin Yuet, A36 Wai Tin Chung Court, Central Tin Fu Court Tin Wah Estate, E37 ⇋ E37 ⇋ Tin Shui Estate, A33/A33X/ E37 ⇋ E37 E37 A33X / Chestwood Court, A34/A36/ E32 / E41 Locwood Court A37 A36 Tin Heng, Tin Yat, Tin Shui A37 ⇋ Hong Kong Wetland A37 ⇋ Wai E37 E37 Park, A37 E32 / E41 A33X / North Vianni Cove A36 Tin Yiu, -

Mainlaying Between Au Tau Treatment Works and Yuen Long Town – Remaining Works
For discussion PWSC(2002-03)23 on 22 May 2002 ITEM FOR PUBLIC WORKS SUBCOMMITTEE OF FINANCE COMMITTEE HEAD 709 - WATERWORKS Water Supplies – Fresh water supplies 227WF – Mainlaying between Au Tau treatment works and Yuen Long Town – remaining works Members are invited to recommend to Finance Committee the upgrading of the remaining part of 227WF to Category A at an estimated cost of $25.7 million in money-of-the-day prices. PROBLEM The existing trunk main between Au Tau treatment works and Yuen Long Town will not be able to cope with anticipated increase in water demand arising from planned developments in the Tuen Mun – Yuen Long Corridor and the north-western part of Tuen Mun by 2005. PROPOSAL 2. The Director of Water Supplies (DWS), with the support of the Secretary for Works, proposes to upgrade the remaining part of 227WF to Category A at an estimated cost of $25.7 million in money-of-the-day (MOD) prices for the laying of fresh water mains in conjunction with the roadworks under 27CG1 “Yuen Long south eastern extension – site formation, roads and drainage works” and 28CG1 “Yuen Long south western extension – site formation, roads and drainage works” along the proposed Road L4 and the existing Ma Tong Road and Ma Tin Road. /PROJECT ..... _____________________________________________________________________________________ 1 A related paper, PWSC(2002-03)29, for the upgrading of 27CG and part of 28CG under Head 707 to Category A is submitted for Members' consideration at this meeting. PWSC(2002-03)23 Page 2 PROJECT SCOPE AND NATURE 3. The scope of works under 227WF comprises the laying of about 5 000 metres (m) of fresh water mains of 1 400 millimetres (mm) in diameter between Au Tau treatment works and Yuen Long Town via the access road of Au Tau treatment works, Kam Tin Road, Castle Peak Road, Yuen Ching Road, Yuen Lung Street, Fung Yau Street East, Fung Yau Street South, the proposed Road L4, Ma Tong Road, Ma Tin Road and Town Park Road South. -
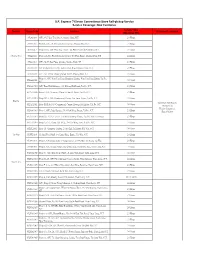
S.F. Express 7-Eleven Convenience Store Self-Pickup Service Service Coverage: New Territories
S.F. Express 7-Eleven Convenience Store Self-pickup Service Service Coverage: New Territories Service Time District Store Code Address Shipment Limitation (Mon-Sun, PH) 852A1003 G/F, 25-27 San Tsoi Street, Sheung Shui, N.T. 24 Hours 852A1005 Shop B, G/F, 76-86 Lung Sum Avenue, Sheung Shui, N.T. 24 Hours 852A1013 Shop 110A, G/F, Ping Hay House, Tai Ping Estate, Sheung Shui, N.T. 24 Hours Sheung Shui 852A1021 Shop 116, Tin Ping Shopping Centre, Tin Ping Estate, Sheung Shiu, NT 24 Hours 852A1016 G/F., No.75 San Fung Avenue, Sheung Shui, NT 24 Hours 852A1020 G/F & Cockloft, No.182 Jockey Club Road, Shueng Shui, N.T. 24 Hours 852A1022 G/F., No. 25 San Cheung Street North, Sheung Shui, N.T. 24 Hours Shop 11, G/F, Wan Tau Tong Shopping Centre, Wan Tau Tong Estate, Tai Po, 852AA1001 24 Hours N.T. 852AA1004 G/F, Tung Fuk Building, 148 Kwong Fuk Road, Tai Po, N.T. 24 Hours 852AA1006 Shop 1, G/F, Greenery Plaza, 3 Chui Yi Street, Tai Po, N.T. 24 Hours 852AA1010 Shop 101 & 109, Commercial Centre, Tai Yuen Estate, Tai Po, N.T. 24 Hours Tai Po Maximum Dimension: 852AA1011 Shop P102 & 103, Commercial Centre, Kwong Fuk Estate, Tai Po, N.T. 24 Hours 36x30x25cm Weight Limitation: 852AA1013 Shop 1, G/F., Jade Garden, No.9 Pak Shing Street, Tai Po, N.T. 24 Hours 5kg or below 852AA1014 Shop No. 227A, Level 2, Tai Wo Shopping Centre, Tai Po, New Territories 24 Hours 852AA1015 Shop A, G/F., Hang Lok Bldg., 2-4 Tai Wing Lane, Tai Po, N.T.