Leaching of Glass-Clay Containers by Ph Effect of Food
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dirección General De Asuntos Religiosos
DIRECTORIO DE ASOCIACIONES RELIGIOSAS DIRECCIÓN GENERAL DE POR ENTIDAD FEDERATIVA ASUNTOS RELIGIOSOS 14 noviembre 2019 Clave SGAR y Asociación Religiosa Domicilio Legal Representante o Apoderado Legal Entidad Federativa: HGO SGAR/1099/93 IGLESIA CRISTIANA INDEPENDIENTE CHAMIZAL NO. 141 COL. EL MIRADOR, TULANCINGO, C.P. 43659, Tel. "AMMI PUEBLO MIO" 9177132716 GRISELDA TELLEZ FERNANDEZ REYNALDO TELLEZ TREJO SGAR/1105/94 2IGLESIA CRISTIANA DE LOS HERMANOS DOMICILIO CONOCIDO, CHICAAVOSCO, ACTOPAN, C.P. 42500, Tel. LIBRES" 9177136335 38071 JOSE SANCHEZ HERRERA LUIS GILBERTO BAUTISTA TAPIA RUBEN CRUZ BAUTISTA SGAR/1111/93 IGLESIA CRISTIANA INDEPENDIENTE CALLE MOCTEZUMA N° 315, COLONIA CENTRO, PACHUCA, C.P. ARMANDO VICENTE VELAZQUEZ AMOR FRATERNAL 42000, Tel. 9177180835 53463 GRANADOS ELIA GOMEZ SANTILLAN GAMALIEL CASTRO CASTILLO JESUS ISAAC ARRIAGA MARTINEZ JORGE PEREZ ANARIO ROSALIO NOLASCO JIMENEZ VICENTE GARCIA SOLARES SGAR/1181/93 MONASTERIO DE CARMELITAS DESCALZAS DE SAN JOSE Y SANTA TERESA EN PROL. 5 DE FEBRERO No.408 PTE., TULANCINGO, C.P. 43600, Tel. MA. DEL CONSUELO RENTERIA TULANCINGO, HGO. 9177530260 Ma. DEL CONSUELO RENTERIA RODRIGUEZ MA. GLORIA REFUGIO AGUILAR CRUZ SGAR/1184/93 MONASTERIO DE SAN FRANCISCO DE MARIANO ESCOBEDO N° 24-68, ACTOPAN, C.P. 42500, Tel. 9177271784 ASIS, EN ACTOPAN, HGO. 7601894 ANA MARIA MARTINEZ ROSALES JUAN VALENTIN BAUTISTA SALINAS RUFINA GACHUZO OLVERA SGAR/13:1118/09 IGLESIA BAUTISTA "PUERTA LA HERMOSA" DE EL BARRIDO, DIOS PADRE EN CALLE CERRADA S/N, COLONIA EL BARRIDO, IXMIQUILPAN, C.P. ISMAEL RUBIO MUÑOZ IXMIQUILPAN, HIDALGO 42300, Tel. 01-773-10-38-663 JOSGE SOLIS BARRERA NOEMI ZUÑIGA GUTIERREZ SGAR/13:141/95 IGLESIA BAUTISTA ELIACIM DE TEPETITLAN, HGO. -

Delegación En El Estado De Hidalgo Subdelegación Agropecuaria Jefatura Del Programa De Fomento a La Agricultura Pa
DELEGACIÓN EN EL ESTADO DE HIDALGO SUBDELEGACIÓN AGROPECUARIA JEFATURA DEL PROGRAMA DE FOMENTO A LA AGRICULTURA PADRÓN DE BENEFICIARIOS DEL COMPONENTE REPOBLAMIENTO Y RECRIA PECUARIA-INFRAESTRUCTURA Y EQUIPO EN LAS UPP MONTO DE NO. ESTADO FOLIO ESTATAL REGION DEL PAIS DISTRITO CADER MUNICIPIO LOCALIDAD BENEFICIO DESCRIPCIÓN TIPO DE PERSONA APOYO ($) 1 HIDALGO HG1600005161 centro HUICHAPAN HUICHAPAN TECOZAUTLA SAN ANTONIO INFRAESTRUCTURA Y EQUIPO DE LAS UPP EQUIPO 280,350.00 FÍSICA 2 HIDALGO HG1600005217 centro TULANCINGO SAN BARTOLO TUTOTEPEC METEPEC EL VESUBIO INFRAESTRUCTURA Y EQUIPO DE LAS UPP INFRAESTRUCTURA 241,455.00 FÍSICA 3 HIDALGO HG1600005232 centro TULANCINGO SAN BARTOLO TUTOTEPEC METEPEC LAS TROJAS INFRAESTRUCTURA 238,994.00 FÍSICA 4 HIDALGO HG1600005388 centro TULANCINGO TULANCINGO SINGUILUCAN SANTA ANA CHICHICUAUTLA INFRAESTRUCTURA Y EQUIPO DE LAS UPP INFRAESTRUCTURA 237,899.20 FÍSICA 5 HIDALGO HG1600005403 centro TULANCINGO TULANCINGO SINGUILUCAN SANTA ANA CHICHICUAUTLA INFRAESTRUCTURA Y EQUIPO DE LAS UPP INFRAESTRUCTURA 237,899.20 FÍSICA 6 HIDALGO HG1600005422 centro HUICHAPAN HUICHAPAN TECOZAUTLA EL SALTO INFRAESTRUCTURA Y EQUIPO DE LAS UPP EQUIPO 196,000.00 FÍSICA 7 HIDALGO HG1600005434 centro HUICHAPAN HUICHAPAN TECOZAUTLA EL SALTO INFRAESTRUCTURA Y EQUIPO DE LAS UPP INFRAESTRUCTURA 18,900.00 FÍSICA 8 HIDALGO HG1600005462 centro MIXQUIAHUALA TULA TULA DE ALLENDE SANTA ANA AHUEHUEPAN INFRAESTRUCTURA Y EQUIPO DE LAS UPP EQUIPO 213,689.70 FÍSICA 9 HIDALGO HG1600005481 centro TULANCINGO TULANCINGO SINGUILUCAN EL VENADO -

Secretaría De Educación Pública De Hidalgo Subdirección De Educación Básica Dirección De Servicios Regionales
SECRETARÍA DE EDUCACIÓN PÚBLICA DE HIDALGO SUBDIRECCIÓN DE EDUCACIÓN BÁSICA DIRECCIÓN DE SERVICIOS REGIONALES DIRECTORIO DE ALMACENES DE SERVICIOS REGIONALES SUBDIRECCIÓ CT TELÉFONO TELÉFONO DIRECCIÓN DE ALMACEN DE LIBROS DIRECCIÓN DE ALMACEN DE ÚTILES NOMBRE RESPONSABLE TELÉFONO No. SUBDIRECTOR CORREO DIRECCIÓN DE OFICINA N REGIONAL OFICINA CELULAR DE TEXTO ESCOLARES ALMACEN CELULAR CARRETERA MÉXICO - TAMPICO (VÍA CORTA), CARRETERA MÉXICO - TAMPICO (VÍA CORTA), PROFR. DAGOBERTO PÉREZ SILVA C. PABLO MARIA ANAYA ESQ. BLVD. ADOLFO HUEJUTLA CHALAHUIYAPA KM. 221 PARQUE HUEJUTLA CHALAHUIYAPA KM. 221 PARQUE 7712203895 PROFR. HERNÁN CORTES 1 HUEJUTLA 13ADG0002R MTRA. ZORAYA INES RIVERA LÓPEZ 01 789 896 3736 771 221 3574 [email protected] LÓPEZ MATEOS S/N, COL. AVIACIÓN CIVÍL C.P. INDUSTRIAL, COL. PARQUE DE POBLAMIENTO, INDUSTRIAL, COL. PARQUE DE POBLAMIENTO, 7712098281 BAUTISTA 43000, HUEJUTLA DE REYES, HGO. HUEJUTLA DE REYES HGO. C.P.43000 HUEJUTLA DE REYES HGO. C.P.43000 7711789560 PROFRA. GRACIELA CISNEROS PUERTA 1 PUERTA 1 CARR. IXMIQUILPAN - ORIZABITA S/N. LOC. CARR. IXMIQUILPAN - ORIZABITA S/N. LOC. LOS CARR. IXMIQUILPAN - ORIZABA S/N. LOC. LOS PROFRA. MA. DEL CARMEN CRUZ 2 IXMIQUILPAN 13ADG0003Q PROFR. CRISTINO URIBE CANO 01 759 723 6199 771 220 9204 [email protected] LOS REMEDIOS C.P. 42300, IXMIQUILPAN 7721028913 REMEDIOS C.P. 42313, IXMIQUILPAN HGO. REMEDIOS C.P. 42313, IXMIQUILPAN HGO. HERNÁNDEZ HGO. AV. MIGUEL HIDALGO NORTE 41 EL LLANO 5574400528 BLVD. TULA - ITURBE 400 COL. ITURBE, C.P. BLVD. TULA - ITURBE 400 COL. ITURBE, C.P. 3 TULA 13ADG0004P PROFR. OCTAVIANO ÁNGELES LÓPEZ 01 773 732 6031 [email protected] 1ra. -

Pachuca De Soto, Hidalgo, a 19 De Junio De 2008
Pachuca de Soto, Hidalgo, a 24 de Octubre de 2016. Apreciable solicitante Presente. En atención y seguimiento a la solicitud de información identificada con el folio número 00232516, realizada por Usted ante la Unidad de Información Pública Gubernamental del Poder Ejecutivo del Estado de Hidalgo, mediante la cual requiere: “¿Cuántos cuerpos sin vida han sido localizados en municipios del estado de Hidalgo desde enero de 2011 hasta agosto de 2016? ¿Cuántos de éstos presentaron signos de tortura? ¿Cuántos fueron hallados en bolsas de plástico, canales de aguas de aguas negras, carreteras o parajes? ¿Cuántos estaban desmembrados? La información, además, debe desagregarse por sexo de las víctimas, años en los que ocurrieron los casos y municipios.” Con la finalidad de dar cumplimiento a lo solicitado, de conformidad con los Artículos 5, Fracción VIII, incisos b) y d), 56 Fracción I, 64, 67 de la Ley de Transparencia y Acceso a la Información Pública Gubernamental para el Estado de Hidalgo y 4 de su Reglamento, la Unidad de Información Pública Gubernamental de éste sujeto obligado se permite hacer de su conocimiento lo referido por la(s) Unidad (es) Administrativa(s) responsable(s) de la información: AVERIGUACIONES PREVIAS 2011 CUERPOS SIN VIDA 61 MUNICIPIOS FRANCISCO I MADERO, EL ARENAL, ACTOPAN, SAN SALVADOR, CD. SAHAGUN, HUEJUTLA, SAN FELIPE ORIZATLAN, TLANCHINOL, IXMIQUILPAN, JACALA, METZTITLAN, LOLOTLA, MIXQUIAHUALA, PACHUCA, APAN, TEPEHUACAN, TIZAYUCA, TULA, AJACUBA, TEPEJI, TENANGO, SAN BARTOLO, CUAUTEPEC, SANTIALGO TULANTEPEC, ACAXOHITLA, -
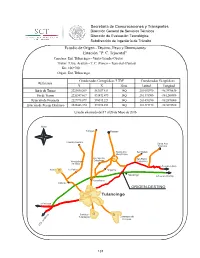
P.C. Tejocotal
Secretaría de Comunicaciones y Transportes Dirección General de Servicios Técnicos Dirección de Evaluación Tecnológica Subdirección de Ingeniería de Tránsito Estudio de Origen - Destino, Peso y Dimensiones Estación ''P. C. Tejocotal'' Carretera: Ent. Tulancingo – Venta Grande (Cuota) Tramo: T. Izq. Acatlán – T. C. (Tlaxco – Tejocotal (Cuota)) Km: 106+700 Origen: Ent. Tulancingo Coordenadas Cartográficas "UTM" Coordenadas Geográficas Referencia Y X Zona Latitud Longitud Inicio de Tramo 2223690.209 563027.611 14Q 20.1092970 -98.3970630 Fin de Tramo 2226947.667 591472.479 14Q 20.1375900 -98.1248090 Estación de Encuesta 2227978.879 574515.221 14Q 20.1476390 -98.2870000 Estación de Pesaje Dinámico 2228443.374 572396.853 14Q 20.1519170 -98.3072500 Estudio efectuado del 17 al 20 de Mayo de 2016 ORIGEN-DESTINO Tulancingo 137 Síntesis del Estudio Origen-Destino, Peso y Dimensiones Estación ''P. C. Tejocotal'' Carretera: Ent. Tulancingo – Venta Grande (Cuota) Lugar: KM 106+700 Origen: Ent. Tulancingo Tramo: T. Izq. Acatlán – T. C. (Tlaxco – Tejocotal (Cuota)) Fecha: Estudio efectuado del 17 al 20 de Mayo de 2016 1.- Volúmenes de Tránsito (Número de Vehículos) Hacia: Venta Grande 18,979 Hacia: Venta Grande Hacia: Ent. Tulancingo Ambos Sentidos Hacia: Ent. Tulancingo 14,682 Promedio Diario 4,745 3,671 8,415 Total Aforado 33,661 Máximo Horario 374 269 628 Máximo Horario Máximo Horario Transito Diario Hacia: Venta Grande A.M. P.M. Hacia: Ent. Tulancingo A.M. P.M. Total Lunes Martes 4,468 281 276 3,654 154 238 8,122 Miércoles 4,091 258 277 3,415 168 216 7,506 Jueves 4,457 316 281 3,702 187 259 8,159 Viernes 5,963 334 374 3,911 192 269 9,874 Sábado Domingo Total 18,979 14,682 33,661 2.- Clasificación Vehicular (Número de Vehículos) Tipo de Vehículo Hacia: Venta Grande Hacia: Ent. -

Evaluation of Comparative Advantages in the Profitability and Competitiveness of the Small-Scale Dairy System of Tulancingo Valley, Mexico
Tropical Animal Health and Production https://doi.org/10.1007/s11250-018-1516-8 REGULAR ARTICLES Evaluation of comparative advantages in the profitability and competitiveness of the small-scale dairy system of Tulancingo Valley, Mexico Rodolfo Rogelio Posadas-Domínguez1 & Oscar Enrique Del Razo-Rodríguez2 & Isaac Almaraz-Buendía2 & Armando Pelaez-Acero2 & Verónica Espinosa-Muñoz2 & Samuel Rebollar-Rebollar3 & Jesús Armando Salinas-Martínez2 Received: 30 August 2017 /Accepted: 17 January 2018 # Springer Science+Business Media B.V., part of Springer Nature 2018 Abstract This article combines a Policy Analysis Matrix with a sensitivity andpovertylineanalysiswiththeobjectiveofevaluatingtheeconomic contribution of comparative advantages to the private profitability and competitiveness of small-scale dairy systems. For 1 year, socioeconomic data were collected from 82 farms selected from four strata via statistical sampling. Two scenarios were established to determine the quantitative contribution of comparative advantages: (1) a simulated scenario, which accounted for the cost of purchasing the total food and the opportunity cost of the family labour force (FLF), and (2) an actual production scenario, which accounted for the cost of producing food and eliminating the payment of the FLF and included other income. The E3 and E4 producers were the most profitable and competitive in the simulated scenario and actual production scenario. Of the four scales evaluated, the E2 and E1 producers were the most efficient in taking advantage of the economic contribution provided by the comparative advantages in their own production of food and employment of the FLF, in addition to accounting for other income, a condition that increased their profitability by 171 and 144% and competitiveness by 346 and 273%, respectively. -

Anexo B. Índices De Intensidad Migratoria México-Estados Unidos Por Entidad Federativa Y Municipio
Anexo B. Índices de intensidad migratoria México-Estados Unidos por entidad federativa y municipio Anexo B. Índices de intensidad migratoria México-Estados Unidos por entidad federativa y municipio Mapa B.1. Aguascalientes: Grado de intensidad migratoria por municipio, 2010 Simbología Grado de intensidad No. de Zacatecas migratoria municipios Muy alto 2 Alto 3 Medio 5 Bajo 1 Muy bajo -- Nulo -- Porcentaje de viviendas en los municipios, según grado de intensidad migratoria 4.9% 6.5% 18.3% 70.2% Medidas descriptivas por indicador Valor Indicador Municipal Estatal asociado a: Min Max Remesas 4.8 3.3 19.7 Emigrantes 2.6 1.6 10.3 Migrantes círculares 1.6 0.9 6.9 Migrantes de retorno 3.1 1.9 11.7 Jalisco 071421Km. Fuente: Estimaciones del CONAPO con base en el INEGI, muestra del diez por ciento del Censo de Población y Vivienda 2010. 67 Consejo Nacional de Población Cuadro B.1. Aguascalientes: Total de viviendas, indicadores sobre migración a Estados Unidos, índice y grado de intensidad migratoria, y lugar que ocupa en los contextos estatal y nacional, por municipio, 2010 Índice de Clave % Viviendas con % Viviendas % Viviendas Lugar que intensidad Lugar que de la Clave del % Viviendas emigrantes a con migrantes con migrantes Índice de Grado de ocupa Entidad federativa/ Total de migratoria ocupa en el entidad munici- que reciben Estados Unidos circulares del de retorno del intensidad intensidad en el Municipio viviendas1 reescalado contexto federa- pio remesas del quinquenio quinquenio quinquenio migratoria migratoria contexto de 0 a estatal3 -

The Teaching of English at Basic Education in the State of Hidalgo
The Teaching of English at Basic Education in the State of Hidalgo: A Case Study Bertha Guadalupe Paredes Zepeda Jovanna Matilde Godínez Martínez Hilda Hidalgo Avilés Norma Angélica Espinosa Butrón1 Marisela Dzul Escamilla (Collaboration) The Secretary of Public Education in Mexico has made several attempts to implement the teaching of English in public primary schools. However, the programs that have been carried out have not reached the desired results. One possible reason for this could be the lack of educational and organized planning to guarantee the appropriateness and feasibility of a project (DíazBarriga F, 2003). The present study led by the University of Sonora is part of a longitudinal study initiated at the end of 2011. The main objective of this study wasto understand the state of the art of English teaching in public schools at basic education level in Mexico. Researchers from the Universities of Baja California, Durango, Guanajuato, Jalisco, Tlaxcala, Guadalajara and Hidalgo participated in this project. In this chapter, information gathered from the program PNIEB in the state of Hidalgo in 2012 and recently updated data is reported. Classroom observations and interviews were carried out for collecting data; the state was divided into three main regions: Sierra Baja and Alta, Valle del Mezquital and Comarca Minera in order to obtain a representative sample of what occurs within the state. Amongst the most relevant findings, it should be mentioned that there is not a specific profile for hiring teachers, a lack of qualified teachers of English, a lack of in-service 1Profesoras investigadoras, Área Académica de Lingüística, Grupo de Investigación de Estudios en Lenguas, Instituto de Ciencias Sociales y Humanidades, Universidad Autónoma del Estado de Hidalgo. -

Infografia HGO COVID-19 Activos.Xlsm
Resumen técnico sobre coronavirus COVID-19 14 de septiembre de 2021 Casos Casos Positivos Defunciones Municipios (v) Activos 14 y 21 días (i) Acumulados (ii) Acumuladas (ii) Mapa de casos activos Pachuca de Soto 220 11,979 1,254 14 días = 1,436 casos 82 municipios Alto riesgo Mineral de la Reforma 95 5,754 475 Tulancingo de Bravo 131 4,467 526 21 días = 0 casos 0 municipios Mediano riesgo Tizayuca 86 4,343 374 Tula de Allende 32 2,837 296 Tepeji del Río de Ocampo 64 2,297 257 Tepeapulco 55 2,123 208 Huejutla de Reyes 31 1,337 162 Apan 40 1,273 147 Zempoala 20 1,213 118 Ixmiquilpan 30 1,075 182 Actopan 28 974 178 Atotonilco de Tula 19 945 119 Huichapan 38 886 64 Tezontepec de Aldama 20 822 114 Santiago Tulantepec de Lugo Guerrero 32 796 78 Cuautepec de Hinojosa 10 739 127 Mixquiahuala de Juárez 15 689 105 Atitalaquia 10 685 87 Tlaxcoapan 23 665 86 San Agustín Tlaxiaca 13 468 94 Zacualtipán de Ángeles 29 448 49 Emiliano Zapata 12 423 48 Tolcayuca 5 410 69 San Salvador 4 406 79 Ajacuba 17 405 49 Progreso de Obregón 12 387 71 Francisco I. Madero 6 373 95 Zapotlán de Juárez 4 371 93 Mineral del Monte 4 350 43 Zimapán 11 343 52 Tlahuelilpan 15 340 57 Epazoyucan 13 336 41 Atotonilco el Grande 2 287 34 Tecozautla 10 287 32 Acaxochitlán 10 277 50 Molango de Escamilla 23 263 11 Chapantongo 7 258 19 Tlanalapa 1 252 30 Chilcuautla 7 249 39 Tenango de Doria 2 224 11 Cardonal 5 214 36 Nopala de Villagrán 20 214 52 Tasquillo 10 210 25 Almoloya 9 207 10 Santiago de Anaya 4 202 7 El Arenal 5 201 32 Acatlán 7 194 29 Tetepango 9 191 19 Alfajayucan 4 177 -

Tropical and Subtropical Agroecosystems E-ISSN: 1870-0462 [email protected] Universidad Autónoma De Yucatán México
Tropical and Subtropical Agroecosystems E-ISSN: 1870-0462 [email protected] Universidad Autónoma de Yucatán México Islas Pelcastre, Margarita; Villagómez Ibarra, José Roberto; Madariaga Navarrete, Alfredo; Castro Rosas, Javier; González Ramirez, César Abelardo; Acevedo Sandoval, Otilio Arturo BIOREMEDIATION PERSPECTIVES USING AUTOCHTHONOUS SPECIES OF Trichoderma sp. FOR DEGRADATION OF ATRAZINE IN AGRICULTURAL SOIL FROM THE TULANCINGO VALLEY, HIDALGO, MEXICO Tropical and Subtropical Agroecosystems, vol. 16, núm. 2, 2013, pp. 265-276 Universidad Autónoma de Yucatán Mérida, Yucatán, México Available in: http://www.redalyc.org/articulo.oa?id=93928324012 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Tropical and Subtropical Agroecosystems, 16 (2013): 265 - 276 BIOREMEDIATION PERSPECTIVES USING AUTOCHTHONOUS SPECIES OF Trichoderma sp. FOR DEGRADATION OF ATRAZINE IN AGRICULTURAL SOIL FROM THE TULANCINGO VALLEY, HIDALGO, MEXICO [PERSPECTIVAS DE BIORREMEDIACIÓN CON Trichoderma sp. NATIVO PARA DEGRADAR ATRAZINA EN SUELO AGRÍCOLA DEL VALLE DE TULANCINGO HIDALGO, MÉXICO] Margarita Islas Pelcastre1,2, José Roberto Villagómez Ibarra.1*, Alfredo Madariaga Navarrete.3, Javier Castro Rosas1, César Abelardo González Ramirez.1 and Otilio Arturo Acevedo Sandoval2 1 Academic Area of Chemistry, Institute of Basic Science and Engineering, Hidalgo State University. City of Knowledge, Pachuca - Tulancingo highway Km 4.5. C. P. 42184. Mineral de la Reforma, Hidalgo, Mexico. Phone: 771 71 7200 0 ext: 2461 and 2902. * E-mail: [email protected] 2 Engineering Academic Area of Agribusiness and Food Engineering, Institute of Agricultural Sciences, Hidalgo State University. -

Ubicación De Cajeros Y Sucursales SANTANDER, Y Presencia a Través De Telecom En HIDALGO
Ubicación de cajeros y sucursales SANTANDER, y Presencia a través de Telecom en HIDALGO TIPO DE LOCALIDAD UBICACIÓN DIRECCION COLONIA C.P. SERVICIO ACAXOTITLÁN TELECOM ACAXOTITLÁN PLAZA JUAREZ SN CENTRO 43720 ACTOPAN CAJERO CM BODEGA ACTOPAN CARR MEXICO - LAREDO KM 120 118-A CENTRO 42500 ACTOPAN CAJERO ACTOPAN HIDALGO NO. 8 CENTRO 42500 ACTOPAN TELECOM ACTOPAN MELCHOR OCAMPO NO. 10 CENTRO 42500 AJACUBA CAJERO AJACUBA PLAZA PRINCIPAL S/N CENTRO 42150 APAN CAJERO APAN PLAZA PRINCIPAL S/N CENTRO 43900 APAN TELECOM APAN PLAZA INDEPENDENCIA SN CENTRO 43900 ATITALAQUIA TELECOM ATITALAQUIA MELCHOR OCAMPO SN CENTRO 42979 ATLAPEXCO CAJERO ATLAPEXCO CORREGIDORA S/N CENTRO 43060 ATLAPEXCO TELECOM ATLAPEXCO DENTRO DEL PALACIO MUNICIPAL CENTRO 43060 ATOTONILCO EL GRANDE TELECOM ATOTONILCO EL GRANDE AV. JORGE VIEZCA PALMA SN CENTRO 43300 ATOTONILCO DE TULA CAJERO PRESTADORA DE SERVICIOS CARR. JOROBAS TULA KM 22.5 CERRITO DEL PARAISO 2 SECCION PROGRESO42980 ATOTONILCO DE TULA CAJERO ATOTONILCO EL GRANDE ZARAGOZA S/N CENTRO 43300 ATOTONILCO DE TULA CAJERO ATOTONILCO DE TULA PLAZA PRINCIPAL S/N CENTRO 52980 CALNALI CAJERO MUNICIPIO CALNALI FELIPE ANGELES NO. 1 CENTRO 43230 CALNALI TELECOM CALNALI PLAZA PRINCIPAL SN CENTRO 43230 CARDONAL CAJERO CARDONAL PLAZA JUAREZ S/N CENTRO 42370 CD. SAHAGUN CAJERO CIUDAD SAHAGUN ALLENDE NO. 10 CENTRO 43990 CD. SAHAGUN TELECOM CD. SAHAGUN PLAZA RODRIGO GOMEZ SN CENTRO 43990 CHAPULHUACAN CAJERO CHAPULHUACAN FRANCISCO SARABIA S/N CENTRO 42281 CHAPULHUACAN TELECOM CHAPULHUACAN FELIPE ANGELES NO. 18 CENTRO 42280 CUAUTEPEC CAJERO MUNICIPIO CUAUTEPEC VENUSTIANO CARRANZA S/N CENTRO 43740 CUAUTEPEC DE HINOJOSA TELECOM CUAUTEPEC DE HINOJOSA JARDIN DEL ARTE NO. 19 CENTRO 43740 EL ARENAL TELECOM EL ARENAL PLAZA PRINCIPAL SN CENTRO 42680 EL MARQUES CAJERO MUNICIPIO DE EPAZOYUCAN AV HIDALGO NO 11 CENTRO 43580 FRANCISCO I. -

The Genetic History of the Otomi in the Central Mexican Valley
University of Pennsylvania ScholarlyCommons Anthropology Senior Theses Department of Anthropology Spring 2013 The Genetic History Of The Otomi In The Central Mexican Valley Haleigh Zillges University of Pennsylvania Follow this and additional works at: https://repository.upenn.edu/anthro_seniortheses Part of the Anthropology Commons Recommended Citation Zillges, Haleigh, "The Genetic History Of The Otomi In The Central Mexican Valley" (2013). Anthropology Senior Theses. Paper 133. This paper is posted at ScholarlyCommons. https://repository.upenn.edu/anthro_seniortheses/133 For more information, please contact [email protected]. The Genetic History Of The Otomi In The Central Mexican Valley Abstract The Otomí, or Hñäñhü, is an indigenous ethnic group in the Central Mexican Valley that has been historically marginalized since before Spanish colonization. To investigate the extent by which historical, geographic, linguistic, and cultural influences shaped biological ancestry, I analyzed the genetic variation of 224 Otomí individuals residing in thirteen Otomí villages. Results indicate that the majority of the mitochondrial DNA (mtDNA) haplotypes belong to the four major founding lineages, A2, B2, C1, and D1, reflecting an overwhelming lack of maternal admixture with Spanish colonizers. Results also indicate that at an intra-population level, neither geography nor linguistics played a prominent role in shaping maternal biological ancestry. However, at an inter-population level, geography was found to be a more influential determinant. Comparisons of Otomí genetic variation allow us to reconstruct the ethnic history of this group, and to place it within a broader-based Mesoamerican history. Disciplines Anthropology This thesis or dissertation is available at ScholarlyCommons: https://repository.upenn.edu/anthro_seniortheses/133 THE GENETIC HISTORY OF THE OTOMI IN THE CENTRAL MEXICAN VALLEY By Haleigh Zillges In Anthropology Submitted to the Department of Anthropology University of Pennsylvania Thesis Advisor: Dr.