33858 Gbn-60-2 Entire Book
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Chapter One – Existing Conditions
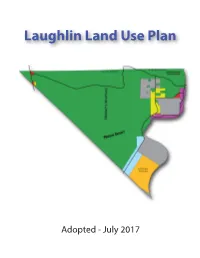
Laughlin Land Use Plan Adopted - July 2017 ACKNOWLEDGEMENTS Clark County Board of Commissioners: Laughlin Town Advisory Board: Steve Sisolak, Chair James Maniaci, Chair Susan Brager, Vice-Chair Kathy Ochs, Vice-Chair Larry Brown Stephanie Bethards Chris Giunchigliani Bruce Henry Marilyn Kirkpatrick Gina Mackey Mary Beth Scow Tammy Harris, Secretary Lawrence Weekly Brian Paulson, County Liaison Office of County Manager: Planning Commission: Yolanda King, Manager Dan Shaw, Chair Randy Tarr, Assistant Manager J. Dapper, Vice-Chair Jeff Wells, Assistant Manager Edward Frasier III Kevin Schiller, Assistant Manager Vivian Kilarski Tom Morley Department of Comprehensive Planning: Nelson Stone Nancy Amundsen, Director Donna Tagliaferrri Community Planning Team: Mario Bermudez, Planning Manager Shane Ammerman, Assistant Planning Manager Kevin Smedley, Principal Planner & Project Lead Paul Doerr, Senior Planner Chris LaMay, GIS Analyst Garrett TerBerg, Principal Planner Michael Popp, Sr. Management Analyst Justin Williams, Parks Planner Ron Gregory, Trails Assistant Planning Manager Scott Hagen, Senior Planner Laughlin Land Use Plan 2017 i ii Laughlin Land Use Plan TABLE OF CONTENTS Introduction ............................................................................................................. 1 State Law ............................................................................................................ 1 Background ......................................................................................................... 1 Purpose -

The Colorado River
KD [Qu THE COLORADO RIVER HISTORY SEVEN-STATES COMPACT AND FUTURE DEVELOPMENT WA WATER RESOURCES CENTER AFlo lCJ,UF?C,IVJESej CENTER i,,nCHIVES DEC 1990 MR a F CALlpnn rvlA OF CALIFORNIA By WALTER GORDON CLARK i THE COLORAI:>O RIVER INDEX Location, Discovery and History . River Characteristics and Formations . Geographical Changes Caused by the Deposit of River Detritus . Development of the Imperial Valley . Appropriation of Waters . The Colorado River Compact . Distribution of Water to the States in the Respective Basins Arizona and the Compact . Present and Ultimate Irrigation Demand in the Upper Basin Present and Ultimate Irrigation Demand in the Lower Basin Reduced Demand After Cultivation . Available Damsites in the Lower Basin . The Boulder Canyon Damsite . Rockfill Type of Dam . Damsites Above Boulder Canyon . Dams Below Boulder Canyon . Balancing Reservoir . Summer Season Power for Irrigation . Equitable Distribution of Cost . Federal Ownership of Water . Federal Ownership of Power . Federal Power Commission Control . Present and Future Demands for Power . A Section of the Grand Canyon from the Rim Showing Extensive Erosion. The Colorado River HE drainage basin of the Colorado River lies between longitude 105 ° 30' west and 116 ° west and latitude 30°40' north and 43°30' north, in Wyoming, Colorado, Utah, Nevada, New Mexico, California, Arizona, T and the extreme northern part of Mexico, meeting tidewater in the Gulf of California at 32'15' north latitude . The distance from the northernmost tributary in Wyoming to the south- ernmost tributary in Mexico is nine hundred miles ; and from its most easterly tributary in Colorado to its most westerly tributary in Nevada is five hundred and fifty . -

Richard E. Lingenfelter, Steamboats on the Colorado River, 1852-1916, University of Arizona Press, Tucson, 1978
@ lglr @ EH gH. e ê3 (-ï @ Õ FE rç-r P @ GÃ e9. t-Ð ô3 eõ- æ @ 5è IA @ @ N9 I A @ @- Steamlboaûs @m the Oonopedo Rflvep 62 flgfl6 Rishand E" Lingenllelûer THE UNIVERSITY OF ARIZONA PRESS TUCSON, ARIZONA About the Author . For permission to use the illustrations contained in this volume we wish to credit the Arizona Department of Library, Archives and Public Richard E. Lingenfelter, a historian by avocation, has been a pro- Records, p. 26; the Arizona Historical Society Library, pp. 25, 28, 39, fessor in residence of geophysics and space physics and astronomy 87, 89, 92-94; The Bancroft Library, pp. 32, 54, 57, 59, 70,79, I78; at the University of Califorrria, Los Angeles, since 1969. He has Barbara Baldwin Ekker, p. 119; the Church Archives Historical Depart- written and edited several books on western American history, in- ment, The Church of Jesus Christ of Latter-day Saints, p. 48; Mrs. cluding First Through the Grand Canyon, The Neusþaþers of Ne- Edwin Wilcox, pp. 107, 116; the Engineering Societies Library, p.77; aada, 1858-1958: A History and, Bibliograþlry, Tlu Songs of the Gold H. E. Huntington Library, San Marino, California, pp. 15, 45, 46,75, Rush, Tlw Songs of the Amerban West, and n 1974The Hardrock Min- 83, 90, 170, 186; Historical Collection, Title Insurance & Trust Co., ers, A History of the Mining Labor Moaement in the Amerban West, San Diego, California, pp. 55, 56, 63, 140; the Map Library, University 1863-1893. of California, Los Angeles, p. 61; the Nevada Historical Society, Reno, pp. -

MASTER HIKE Filecomp
Edition 2: December 22, 2011 SCPD HIKES LISTED BY DIFFICULTY Key: ABSP: Anza-Borrego State Park; CVP: Coachella Valley Preserve; JTNP: Joshua Tree National Park; PCT: Pacific Crest Trail EASY Bear Creek Trail Butler-Abrams Trail CVP, Biskra Palms Oasis CVP, Guided Tour, Sand Dunes CVP, McCallum Grove Oasis Earl Henderson Trail Indian Canyon Sampler Indio Hills Walkabout, I Indio Hills Walkabout, II JTNP, Barker Dam and Wall Street Mill JTNP, Keys Ranch Tour & Hidden Valley Hike JTNP, Pine City Plus Living Desert Private Walking Tour Lower Palm Canyon Mecca Hills, Big Utah Canyon Mecca Hills, Little Painted Canyon Walkabout Morrow Trail (Beginning) La Quinta Cove Loop Oak Glen, Redwoods, Tri-Tip Sandwich & Apple Pie Randall Henderson Trail Salton Sea Bat Caves Sonny Bono Refuge Center, Obsidian Butte & Mud Volcanoes Tahquitz Canyon Waterfalls Trail Whitewater Preserve: Wine & Wildflowers EASY/MODERATE ABSP, Borrego Palm Canyon Bear Canyon Big Morongo Canyon Preserve Bump & Grind (Beginner’s) Carrizo Canyon CVP, Bee Rock Mesa Ridge CVP, Hidden Palms CVP, Moon Rock Trail & Canyon Wash Loop CVP, Willis Palms Trail Ernie Maxwell Scenic Trail Long Valley Hike Mecca Hills, Big Split Rock/ Slot Canyon Walkabout Mecca Hills, Little Painted Canyon Walkabout Whitewater Canyon View Loop MODERATE ABSP, Calcite Mine Big Morongo Canyon (One Way) Bump & Grind/ Herb Jeffries/ Mike Schuler Loop Bump & Grind (by Moonlight) CVP, Bee Rock Mesa & Pushawalla Canyon CVP, Herman’s Peak Hike CVP, Horseshoe Palms Hike CVP, Pushawalla Canyon Eisenhower Peak Loop, -

River Cities VISITOR & RELOCATION GUIDE
DINING REAL ESTATE RECREATION EDUCATION DEVELOPMENT HEALTH Relocating to the River Cities VISITOR & RELOCATION GUIDE Bullhead City | Laughlin | Fort Mohave | Mohave Valley | Needles Relocating to the EXPERIENCE River Cities AWARD-WINNING EXCITEMENT! Best Casino PLAY – Casino Player Magazine Best Hotel Best Overall Gaming Resort STAY – Casino Player Magazine Best Overall Dining DINE – Casino Player Magazine CALL TODAY TO MAKE YOUR RESERVATION AND WE’LL WELCOME YOU TO THE RIVER 800.950.7700 GOLDENNUGGET.COM Relocating to the River Cities VISITOR & RELOCATION GUIDE CONTENTS DINING 4 REAL ESTATE 8 RECREATION 12 EDUCATION 22 DEVELOPMENT 28 HEALTH 32 Relocating to the River Cities VISITOR & RELOCATION GUIDE Relocating to the River Cities LARRY KENDRICK General Manager | WELLS ANDREWS Sales/Circulation Director BILL MCMILLEN Editorial | ERIC FRAKES Operations Manager | JASON LORD Layout & Design ADVERTISING: Jody Bristyan, CAREY FEARING, JAMIE MCCORKLE, NANCY Novak, LU WEISS PRODUCTION: BEN KANE Prepress Manager, MICHAEL KENITZER Relocating to the River Cities is published and distributed annually. The Bullhead Area Chamber of Commerce contributed to this magazine and will make the guide available online and at their local office. Call the chamber at (928) 754-4121 to request by mail. Although every attempt is to be as accurate as possible, News West Publishing is not responsible for any errors, misprints, omissions, or accuracy of the stories in this publication. ©2019 News West Publishing, Inc News West Publishing | 2435 Miracle Mile, P.O. Box 21209, Bullhead City, AZ 86442 | 928.763.2505 | www.MohaveDailyNews.com 3 DINING The River Cities are home to a wide variety of fantastic restaurants suited for any taste. -

Mojave Miocene Robert E
Mojave Miocene Robert E. Reynolds, editor California State University Desert Studies Center 2015 Desert Symposium April 2015 Front cover: Rainbow Basin syncline, with rendering of saber cat by Katura Reynolds. Back cover: Cajon Pass Title page: Jedediah Smith’s party crossing the burning Mojave Desert during the 1826 trek to California by Frederic Remington Past volumes in the Desert Symposium series may be accessed at <http://nsm.fullerton.edu/dsc/desert-studies-center-additional-information> 2 2015 desert symposium Table of contents Mojave Miocene: the field trip 7 Robert E. Reynolds and David M. Miller Miocene mammal diversity of the Mojave region in the context of Great Basin mammal history 34 Catherine Badgley, Tara M. Smiley, Katherine Loughney Regional and local correlations of feldspar geochemistry of the Peach Spring Tuff, Alvord Mountain, California 44 David C. Buesch Phytoliths of the Barstow Formation through the Middle Miocene Climatic Optimum: preliminary findings 51 Katharine M. Loughney and Selena Y. Smith A fresh look at the Pickhandle Formation: Pyroclastic flows and fossiliferous lacustrine sediments 59 Jennifer Garrison and Robert E. Reynolds Biochronology of Brachycrus (Artiodactyla, Oreodontidae) and downward relocation of the Hemingfordian– Barstovian North American Land Mammal Age boundary in the respective type areas 63 E. Bruce Lander Mediochoerus (Mammalia, Artiodactyla, Oreodontidae, Ticholeptinae) from the Barstow and Hector Formations of the central Mojave Desert Province, southern California, and the Runningwater and Olcott Formations of the northern Nebraska Panhandle—Implications of changes in average adult body size through time and faunal provincialism 83 E. Bruce Lander Review of peccaries from the Barstow Formation of California 108 Donald L. -

Chapter 3 Affected Environment
Chapter Three Chapter 3 Affected Environment 3.1 Introduction Chapter 3 describes environmental resources (e.g., hydrologic, biologic, and socioeconomic) of the Colorado River Basin that could be affected by the proposed federal action and the range of alternatives for implementing the proposed federal action described in Chapter 1 and Chapter 2, respectively. The extent to which each specific resource may be impacted is discussed in Chapter 4. Section 3.2 presents a general discussion of the geographic scope within which potential effects of the alternatives are analyzed, and describes each of the potentially affected Colorado River reaches and water service areas. Subsequent sections in this chapter describe specific resources that may be potentially affected, such as water deliveries, recreation and biologic resources. Each resource section contains a discussion of one or more specific issues identified for consideration through scoping, public review and comment, and internal review (Chapter 1, Table 1.5-1). Final EIS – Colorado River Interim Guidelines for Lower Basin Shortages and Coordinated Operations for 3-1 October 2007 Lake Powell and Lake Mead Affected Environment Chapter 3 This page intentionally left blank. Final EIS – Colorado River Interim Guidelines for October 2007 3-2 Lower Basin Shortages and Coordinated Operations for Lake Powell and Lake Mead Chapter 3 Affected Environment 3.2 Geographic Scope The proposed federal action considers modified operations of Lake Powell and Lake Mead over a wide range of reservoir elevations as addressed by the four operational elements discussed in Section 1.2, i.e., shortage conditions, coordinated operations of Lake Powell and Lake Mead, storage and delivery of Colorado River system and non-system water, and the modified ISG. -

Biota of Lake Mead: Annotated Checklist and Bibliography
Publications (WR) Water Resources 3-1977 Biota of Lake Mead: Annotated checklist and bibliography Wesley E. Niles University of Nevada, Las Vegas Charles L. Douglas University of Nevada, Las Vegas National Park Service Follow this and additional works at: https://digitalscholarship.unlv.edu/water_pubs Part of the Natural Resources and Conservation Commons, and the Natural Resources Management and Policy Commons Repository Citation Niles, W. E., Douglas, C. L., National Park Service (1977). Biota of Lake Mead: Annotated checklist and bibliography. Lake Mead Report Series, Project report NO.1 Available at: https://digitalscholarship.unlv.edu/water_pubs/7 This Technical Report is protected by copyright and/or related rights. It has been brought to you by Digital Scholarship@UNLV with permission from the rights-holder(s). You are free to use this Technical Report in any way that is permitted by the copyright and related rights legislation that applies to your use. For other uses you need to obtain permission from the rights-holder(s) directly, unless additional rights are indicated by a Creative Commons license in the record and/or on the work itself. This Technical Report has been accepted for inclusion in Publications (WR) by an authorized administrator of Digital Scholarship@UNLV. For more information, please contact [email protected]. Biota of Lake Mead National Recreation Area NEVADA - ARIZONA Lake Mead Report Series UNITED STATES DEPARTMENT OF THE INTERIOR - NATIONAL PARK SERVICE DELAMQSt COOPERATIVE NATIONAL PARK RESOURCES STUDIES UNIT UNIVERSITY OF NEVADA/LAS VEGAS Department of Biological Sciences Las Vegas, Nevada 89153 Charles L. Douglas, Unit Leader Senior Research Scientist National Park Service BIOTA OF LAKE MEAD NATIONAL RECREATION AREA PROJECT REPORT NO. -

Chapter 3 Affected Environment
1 Chapter Three 2 Affected Environment This page intentionally left blank. Chapter 3 Affected Environment 1 3.1 Introduction 2 Chapter 3 describes environmental resources (i.e., hydrologic, biologic, and socioeconomic) of 3 the Colorado River Basin that could be affected by the proposed federal action described in 4 Chapter 1and Chapter 2. The extent to which each specific resource may be impacted is 5 discussed in Chapter 4. 6 Section 3.2 presents a general discussion of the geographic scope within which potential effects 7 of the alternatives are analyzed and describes each of the Colorado River reaches and affected 8 water service areas. Subsequent sections in this chapter describe specific resources that may be 9 potentially affected, such as water deliveries, recreation and biologic resources. Each resource 10 section contains a discussion of one or more specific issues identified for consideration through 11 scoping, public review and comment, and internal review (Chapter 1, Table 1.5-1). Draft EIS – Colorado River Interim Guidelines for Lower Basin Shortages and Coordinated Operations 3-1 February 2007 for Lake Powell and Lake Mead Affected Environment Chapter 3 1 This page intentionally left blank. Draft EIS – Colorado River Interim Guidelines for February 2007 3-2 Lower Basin Shortages and Coordinated Operations for Lake Powell and Lake Mead Chapter 3 Affected Environment 1 3.2 Geographic Scope 2 The proposed federal action considers modified operations of Lake Powell and Lake Mead over 3 a wide range of reservoir elevations as addressed by the four operational elements discussed in 4 Section 1.2: shortage conditions; coordinated operation of Lake Powell and Lake Mead; storage 5 and delivery of Colorado River system and non-system water; and the modified ISG. -

Environmental Impact Statement
APPENDIX A SCOPING REPORT WEST MOJAVE ROUTE NETWORK PROJECT Scoping Report U.S. Department of the Interior Bureau of Land Management California Desert District Office 22835 Calle San Juan De Los Lagos Moreno Valley, CA 92553 June 2012 This page intentionally left blank. Scoping Report Table of Contents TABLE OF CONTENTS 1.0 Introduction ..........................................................................................................................1 1.1 Purpose and Need for the West Mojave Route Network Project ......................................... 5 1.2 Planning Criteria ..................................................................................................................... 5 2.0 Scoping Process .....................................................................................................................6 2.1 Purpose of Public and Agency Seeping .................................................................................. 6 2.2 Seeping Framework and Agency Consultation ................... .. ................................................. 6 2.3 Purpose of Seeping Report ..................................................................................................... 7 2.4 Notification and Seeping Meeting Advertisements ............................................................... 7 2.5 Seeping Meetings ................................................................................................................... 7 3.0 Scoping Comments ............................................................................................................. -

The History of Davis
DAVIS DAM Looking north during construction of Davis Dam. Arizona is on the right side and Nevada to the left. In the foreground are the temporary construction bridges. Demolished after the completion of the dam, there would not be another bridge built until 1987 when Don Laughlin built a public bridge downstream near his Riverside Casino. Davis Dam was constructed from 1942 to 1952 as the third and final impoundment of the lower Colorado River by the Bureau of Reclamation. Together with Hoover Dam 67 miles upstream and Parker Dam 88 miles downstream, Davis Dam was built to provide flood protection, hydroelectric generation, and water storage for agricultural, industrial, and domestic use in the Southwest. Storage of water behind the dam and the regulation of water flow in the Colorado River below the dam allow the United States to comply with the Mexican Treaty of 1944, particularly annual delivery of 1.5 million acre-feet of water to Mexico in the Colorado River. Davis Dam was the only major dam in which construction included excavation of a new river channel, a portion of which became a through-dam forebay for delivery of water to the power plant and spillway. Davis Dam is a rock and earth-fill gravity dam rising 200' above the lowest point of its foundation and 140' above the level of the Colorado River. The dam crest is 1,600' long between the Arizona and Nevada sides of Pyramid Canyon, and the 50' wide dam crest accommodates a 1 convergence of Arizona State Highway 68 and Nevada State Highway 163. -

Entertainer.Com | Wednesday-Tuesday, Dec
LAKE 3 MOHAVE LAUGHLIN, NEVADA Pyramid Canyon KATHERINE Day Use Area LANDING 16-22, 2020 DEC. | WEDNESDAY-TUESDAY, LAUGHLINENTERTAINER.COM DAVIS DAM HWY 163 Davis Dam Rd HWY TO LAS VEGAS GREENWAY PARK TRAIL DAVIS CAMP 68 entertainer & PED. BRIDGE LAUGHLIN WEDNESDAY-TUESDAY, DEC. 16-22, 2020 BRIDGE TO KINGMAN FISHERMAN'S ACCESS AND I-40 A CIVIC DR. 1 B AREA THOMAS EDISON DR. C 2 10 3 MAP D H CASINO DR.4 BRUCE 10 WOODBURY DR. 5 HWY 95 LAUGHLIN, NV 6 7 8 BULLHEAD PARKWAY BULLHEAD 9 BULLHEAD COMMUNITY E PARK J LAUGHLIN BAY SILVER CREEK WARMC HOSPITAL 6 MARINA I NEEDLES HIGHWAYBIG BEND HANCOCK BLVD. RAMAR RD. POINTS OF K 4 12 PROMOTIONS MARINA BLVD. INTEREST LAZY RIVERVIEW DR. N. OATMAN RD. HARRY'S F ROTARY 6 JEROME RIVIERA PARK L MARINA BULLHEAD CITY, AZ 15 POKER PLAYING 7 THE EDGES AHA MACAV PARKWAY MOHAVE COMMUNITY COLLEGE 16 JACKPOTS G N 8 DINING GUIDE 10 PRESCOTT 18 MOVIES MAP NOT TO SCALE HWY 95 ATTENTION M AZTEC RD. TO GOLF COURSES 11 VALLEY VIEW Huukan Golf Club (off Joy Lane) Masks are required in all public places, including inside casinos, HOSPITAL El Rio (near Boundary Cone Rd.) in Nevada until further notice, as ordered by Gov. Steve Sisolak. VETERANS SPIRIT 12 Los Lagos (on Boundary Cone Rd.) MEMORIAL MOUNTAIN I BRIDGE 9 MILES SOUTH Rivers Edge (Needles) MAP KEY CASINO CONTACT INFORMATION BOATING CASINO GOLF MISCELLANEOUS HOSPITAL DOCK COURSE The following local numbers are the best way to reach the Laughlin resorts for more information.