Hemiptera: Coreidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Review of Acanthocephala (Hemiptera: Heteroptera: Coreidae) of America North of Mexico with a Key to Species
Zootaxa 2835: 30–40 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) Review of Acanthocephala (Hemiptera: Heteroptera: Coreidae) of America north of Mexico with a key to species J. E. McPHERSON1, RICHARD J. PACKAUSKAS2, ROBERT W. SITES3, STEVEN J. TAYLOR4, C. SCOTT BUNDY5, JEFFREY D. BRADSHAW6 & PAULA LEVIN MITCHELL7 1Department of Zoology, Southern Illinois University, Carbondale, Illinois 62901, USA. E-mail: [email protected] 2Department of Biological Sciences, Fort Hays State University, Hays, Kansas 67601, USA. E-mail: [email protected] 3Enns Entomology Museum, Division of Plant Sciences, University of Missouri, Columbia, Missouri 65211, USA. E-mail: [email protected] 4Illinois Natural History Survey, University of Illinois at Urbana-Champaign, Illinois 61820, USA. E-mail: [email protected] 5Department of Entomology, Plant Pathology, & Weed Science, New Mexico State University, Las Cruces, New Mexico 88003, USA. E-mail: [email protected] 6Department of Entomology, University of Nebraska-Lincoln, Panhandle Research & Extension Center, Scottsbluff, Nebraska 69361, USA. E-mail: [email protected] 7Department of Biology, Winthrop University, Rock Hill, South Carolina 29733, USA. E-mail: [email protected] Abstract A review of Acanthocephala of America north of Mexico is presented with an updated key to species. A. confraterna is considered a junior synonym of A. terminalis, thus reducing the number of known species in this region from five to four. New state and country records are presented. Key words: Coreidae, Coreinae, Acanthocephalini, Acanthocephala, North America, review, synonymy, key, distribution Introduction The genus Acanthocephala Laporte currently is represented in America north of Mexico by five species: Acan- thocephala (Acanthocephala) declivis (Say), A. -

Squash Bug, Anasa Tristis (Degeer)1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. EENY-077 Squash Bug, Anasa tristis (DeGeer)1 John L. Capinera2 Introduction Egg The squash bug, Anasa tristis, attacks cucurbits Eggs are deposited on the lower surface of (squash and relatives) throughout Central America, leaves, though occasionally they occur on the upper the United States, and southern Canada. Several surface or on leaf petioles. The elliptical egg is related species in the same genus coexist with squash somewhat flattened and bronze in color. The average bug over most of its range, feeding on the same plants egg length is about 1.5 mm and the width about 1.1 but causing much less injury. mm. Females deposit about 20 eggs in each egg cluster. Eggs may be tightly clustered (Figure 1) or Life Cycle spread a considerable distance apart, but an equidistant spacing arrangement is commonly The complete life cycle of squash bug commonly observed. Duration of the egg stage is about seven to requires six to eight weeks. Squash bugs have one nine days. generation per year in northern climates and two to three generations per year in warmer regions. In Nymph intermediate latitudes the early-emerging adults from the first generation produce a second generation There are five nymphal instars. The nymphal whereas the late-emerging adults go into diapause. stage requires about 33 days for complete Both sexes overwinter as adults. The preferred development. The nymph is about 2.5 mm in length overwintering site seems to be in cucurbit fields when it hatches, and light green in color. -

Jordan Beans RA RMO Dir
Importation of Fresh Beans (Phaseolus vulgaris L.), Shelled or in Pods, from Jordan into the Continental United States A Qualitative, Pathway-Initiated Risk Assessment February 14, 2011 Version 2 Agency Contact: Plant Epidemiology and Risk Analysis Laboratory Center for Plant Health Science and Technology United States Department of Agriculture Animal and Plant Health Inspection Service Plant Protection and Quarantine 1730 Varsity Drive, Suite 300 Raleigh, NC 27606 Pest Risk Assessment for Beans from Jordan Executive Summary In this risk assessment we examined the risks associated with the importation of fresh beans (Phaseolus vulgaris L.), in pods (French, green, snap, and string beans) or shelled, from the Kingdom of Jordan into the continental United States. We developed a list of pests associated with beans (in any country) that occur in Jordan on any host based on scientific literature, previous commodity risk assessments, records of intercepted pests at ports-of-entry, and information from experts on bean production. This is a qualitative risk assessment, as we express estimates of risk in descriptive terms (High, Medium, and Low) rather than numerically in probabilities or frequencies. We identified seven quarantine pests likely to follow the pathway of introduction. We estimated Consequences of Introduction by assessing five elements that reflect the biology and ecology of the pests: climate-host interaction, host range, dispersal potential, economic impact, and environmental impact. We estimated Likelihood of Introduction values by considering both the quantity of the commodity imported annually and the potential for pest introduction and establishment. We summed the Consequences of Introduction and Likelihood of Introduction values to estimate overall Pest Risk Potentials, which describe risk in the absence of mitigation. -

Hemiptera: Heteroptera) of Magallanes Region: Checklist and Identification Key to the Species
Anales Instituto Patagonia (Chile), 2016. Vol. 44(1):39-42 39 The Coreoidea Leach, 1815 (Hemiptera: Heteroptera) of Magallanes Region: Checklist and identification key to the species Los Coreoidea Leach, 1815 (Hemiptera: Heteroptera) de la Región de Magallanes: Lista de especies y clave de identificación Eduardo I. Faúndez1,2 Abstract Slater, 1995), and several species are economically Members of the Coreoidea of Magallanes Region important; there are, however, also cases in which are listed. First records in the Magallanes Region are species of this superfamily have been recorded provided for Harmostes (Neoharmostes) procerus feeding on carrion and dung (Mitchell, 2000). Berg, 1878 and Althos nigropunctatus (Signoret, Additionally, biting humans has been recorded 1864). It is concluded that three species classified in members of this group (Faúndez & Carvajal, in three genera and two families are present in the 2011). In Chile, the Coreoidea is represented by region. A key to the species is provided. two families, the Coreidae and Rhopalidae, and the major diversity for this group is found in the central Key words: Coreidae, Rhopalidae, Distribution, zone of the country (Faúndez, 2015b). New records, Chile. In Magallanes, very little is known about the species of this superfamily, and actually there is Resumen only one species officially recorded from the area: Se listan los Coreoidea de la Region de Magallanes. the dunes bug, Eldarca nigroscutellata Faúndez, Se entregan los primeros registros para la región 2015 (Coreidae). The purpose of this contribution de Harmostes (Neoharmostes) procerus Berg, is to provide an update of this group in the 1878 y Althos nigropunctatus (Signoret, 1864). -

A Catalogue of the Type Specimens of Heteroptera (Insecta) Housed at the Instituto Fundación Miguel Lillo (Tucumán, Argentina)
Revista de la Sociedad Entomológica Argentina ISSN: 0373-5680 ISSN: 1851-7471 [email protected] Sociedad Entomológica Argentina Argentina A catalogue of the type specimens of Heteroptera (Insecta) housed at the Instituto Fundación Miguel Lillo (Tucumán, Argentina) MELO, María C.; ZAMUDIO, María P.; DELLAPÉ, Pablo M. A catalogue of the type specimens of Heteroptera (Insecta) housed at the Instituto Fundación Miguel Lillo (Tucumán, Argentina) Revista de la Sociedad Entomológica Argentina, vol. 77, no. 2, 2018 Sociedad Entomológica Argentina, Argentina Available in: https://www.redalyc.org/articulo.oa?id=322054935004 PDF generated from XML JATS4R by Redalyc Project academic non-profit, developed under the open access initiative Artículos A catalogue of the type specimens of Heteroptera (Insecta) housed at the Instituto Fundación Miguel Lillo (Tucumán, Argentina) Catálogo de los tipos de Heteroptera (Insecta) depositados en el Instituto Fundación Miguel Lillo (Tucumán, Argentina) María C. MELO [email protected] Universidad Nacional de La Plata, CONICET, Argentina María P. ZAMUDIO Fundación Miguel Lillo, Argentina Pablo M. DELLAPÉ Universidad Nacional de La Plata, CONICET, Argentina Revista de la Sociedad Entomológica Argentina, vol. 77, no. 2, 2018 Abstract: is catalogue contains information about the type material of the suborder Sociedad Entomológica Argentina, Heteroptera housed at the Entomological Collection of the Instituto Fundación Argentina Miguel Lillo (IFML-Tucumán, Argentina). We listed 60 holotypes and 453 paratypes Received: 11 October 2017 belonging to 20 families, and three species and one subspecies that were not found in the Accepted: 04 May 2018 collection but, according the original description, should be deposited in IFML. Finally, Published: 28 May 2018 we listed 15 species that are labeled and coded as types but that are no part of the original type series. -

Lepidoptera:Pyralidae) in Florida
Mississippi State University Scholars Junction Theses and Dissertations Theses and Dissertations 1-1-2009 The Ecology of Cactoblastis Cactorum (Berg) (Lepidoptera:pyralidae) in Florida Kristen Erica Sauby Follow this and additional works at: https://scholarsjunction.msstate.edu/td Recommended Citation Sauby, Kristen Erica, "The Ecology of Cactoblastis Cactorum (Berg) (Lepidoptera:pyralidae) in Florida" (2009). Theses and Dissertations. 4323. https://scholarsjunction.msstate.edu/td/4323 This Graduate Thesis - Open Access is brought to you for free and open access by the Theses and Dissertations at Scholars Junction. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of Scholars Junction. For more information, please contact [email protected]. THE ECOLOGY OF CACTOBLASTIS CACTORUM (BERG) (LEPIDOPTERA: PYRALIDAE) IN FLORIDA By Kristen Erica Sauby A Thesis Submitted to the Faculty of Mississippi State University in Partial Fulfillment of the Requirements for the Degree of Master of Science in Biological Sciences in the Department of Biological Sciences Mississippi State, Mississippi August 2009 Copyright by Kristen Erica Sauby 2009 THE ECOLOGY OF CACTOBLASTIS CACTORUM (BERG) (LEPIDOPTERA: PYRALIDAE) IN FLORIDA By Kristen Erica Sauby Approved: Christopher P. Brooks Richard L. Brown Assistant Professor of Biological Sciences Professor of Entomology (Director of Thesis) (Committee Member) Gary N. Ervin Gary N. Ervin Associate Professor of Biological Sciences Graduate Coordinator of the -

Squash Bug, Anasa Tristis (Degeer)
CHARACTERIZING THE OVERWINTERING AND El\ffiRGENCE BERAVIORS OF THE ADULT SQUASH BUG, ANASA TRISTIS (DEGEER) By JESSE ALAN EIBEN Bachelor of Science in Psychobiology Albright College Reading, PA 2001 Submitted to the Faculty of the Graduate College of Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE December, 2004 CHARACTERIZING THE OVERWINTERING AND EMERGENCE BEHAVIORS OF THE ADULT SQUASH BUG, ANASA TRISTIS (DEGEER) Thesis Approved: I Thesis Advisor 7Dean of the Graduate College ii PREFACE Research was conducted from 2002 to 2004 at the Wes Watkins Agricultural Research and Extension Center (WWAREC) in Lane, Oklahoma to illuminate the specific behaviors of adult overwintering squash bugs during the winter hibernating period and during their spring emergence. These studies were conducted in the field with the adult squash bug, Anasa tristis (Degeer), its host plant the yellow crook-necked squash, Cucurbita pepo 'lemondrop', and its overwintering habitats consisting of many sheltering objects found in the ecological landscape. The first chapter is introductory and the last two chapters present results as complete manuscripts to be submitted to scientific journals following manuscript guidelines established by the Entomological Society of America. I would like to acknowledge the following people for valuable advice and assistance throughout my research endeavors at OSU. My sincerest thanks go to my major advisor Dr. Jonathan Edelson. He has given me this wonderful opportunity and the freedom to pursue a project that was both wide in berth and exploratory in nature. The other members of my graduate committee, Dr. Kris Giles, Dr. Thomas Phillips, and Dr. -

Anasa Tristis) Can Be a Serious Insect Pest for Organic Summer Squash Growers
EFFECTS OF COVER CROPS AND ORGANIC INSECTICIDES ON SQUASH BUG (ANASA TRISTIS) POPULATIONS by LINDSAY NICHOLE DAVIES (Under the Direction of David Berle) ABSTRACT Squash bugs (Anasa tristis) can be a serious insect pest for organic summer squash growers. The purpose of this research was to evaluate two methods to control A. tristis populations. The first experiment involved planting cover crops adjacent to summer squash in an effort to attract natural enemies to keep A. tristis populations in check. Natural enemies were attracted to the plots, but did not significantly reduce A. tristis populations. This may have been due to other food sources in the plots, such as pollen, nectar, and aphids. Also, summer squash yields were negatively affected by the cover crop treatments. The second experiment involved evaluating the efficacy of organic insecticides on A. tristis adults and nymphs. Results of this study showed pyrethrin-based sprays are best for controlling A. tristis. INDEX WORDS: Summer squash, Diversified planting, Natural enemies, Pesticides, Organic agriculture, Sustainable agriculture, Biological control EFFECTS OF COVER CROPS AND ORGANIC INSECTICIDES ON SQUASH BUG (ANASA TRISTIS) POPULATIONS by LINDSAY NICHOLE DAVIES B.S., Indiana University, 2011 A Thesis Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE ATHENS, GEORGIA 2016 © 2016 Lindsay Nichole Davies All Rights Reserved EFFECTS OF COVER CROPS AND ORGANIC INSECTICIDES ON SQUASH BUG (ANASA TRISTIS) POPULATIONS by LINDSAY NICHOLE DAVIES Major Professor: David Berle Committee: Paul Guillebeau Elizabeth Little Electronic Version Approved: Suzanne Barbour Dean of the Graduate School The University of Georgia May 2016 DEDICATION This thesis is dedicated to my friends, family, and fiancé. -

Catorhintha Schaffneri (Coreidae), a New Biological Control Agent for Pereskia Aculeata (Cactaceae)
1 Post-Release Evaluation and Thermal Physiology of the Pereskia Stem-Wilter, Catorhintha schaffneri (Coreidae), a New Biological Control Agent for Pereskia aculeata (Cactaceae) RHODES UNIVERSITY Where leaders learn THESIS Submitted in fulfilment of the requirements for the degree MASTERS OF SCIENCE at Rhodes University By Phillippa Claire Muskett February 2017 ii Abstract Catorhintha schaffneri Brailovsky and Garcia (Hemiptera: Coreidae) is a biological control agent that was recently accepted for release in South Africa to control Pereskia aculeata Miller (Cactaceae), an invasive creeping cactus. The aim of this thesis was to conduct post-release research to ensure that C. schaffneri is utilised to its full potential. To achieve this aim, and focus release efforts, the thermal physiology of C. schaffneri was investigated to predict where in South Africa the agent is most likely to establish. These predictions were then tested by releasing the agent at field sites with a wide variety of climatic conditions and evaluating establishment success. When invasive plants invade a wide distribution, made up of areas with different climatic conditions, biological control agents may not establish or be effective throughout the invaded distribution. According to the thermal physiology of C. schaffneri, it is most likely to establish and become effective in the subtropical region of South Africa, along the coast of KwaZulu- Natal. Cold winters, or generally low year-round temperatures, may limit establishment in the more temperate areas of South Africa in the Eastern and Western Cape as well as inland in the Highveld region. These predictions can be used to focus release efforts to climatically suitable regions and stop releases in areas where C. -

Two Coreidae (Hemiptera), Chelinidea Vittiger and Anasa Armigera, New for Arkansas, U.S.A. Stephen W
Journal of the Arkansas Academy of Science Volume 62 Article 23 2008 Two Coreidae (Hemiptera), Chelinidea vittiger and Anasa armigera, New for Arkansas, U.S.A. Stephen W. Chordas III The Ohio State University, [email protected] Peter W. Kovarik Columbus State Community College Follow this and additional works at: http://scholarworks.uark.edu/jaas Part of the Entomology Commons Recommended Citation Chordas, Stephen W. III and Kovarik, Peter W. (2008) "Two Coreidae (Hemiptera), Chelinidea vittiger and Anasa armigera, New for Arkansas, U.S.A.," Journal of the Arkansas Academy of Science: Vol. 62 , Article 23. Available at: http://scholarworks.uark.edu/jaas/vol62/iss1/23 This article is available for use under the Creative Commons license: Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0). Users are able to read, download, copy, print, distribute, search, link to the full texts of these articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This General Note is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Journal of the Arkansas Academy of Science by an authorized editor of ScholarWorks@UARK. For more information, please contact [email protected]. Journal of the Arkansas Academy of Science, Vol. 62 [2008], Art. 23 Two Coreidae (Hemiptera), Chelinidea vittiger and Anasa armigera, New for Arkansas, U.S.A. S. Chordas III1 and P. Kovarik2 1Center for Life Sciences Education, The Ohio State University, 260 Jennings Hall, 1735 Neil Avenue, Columbus, Ohio 43210 2 Columbus State Community College 239 Crestview Road, Columbus, Ohio 43202 1Correspondence: [email protected] Most leaf-footed bugs (Hemiptera: Coreidae) Herring (1980) and Froeschner (1988) in not occurring north of Mexico are essentially generalist recognizing subspecies of Chelinidea vittiger (which phytophagous insects feeding on tender shoots or were almost solely based on color variations). -
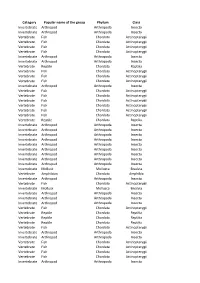
Category Popular Name of the Group Phylum Class Invertebrate
Category Popular name of the group Phylum Class Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate Reptile Chordata Reptilia Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Fish Chordata Actinopterygii Vertebrate Reptile Chordata Reptilia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Mollusk Mollusca Bivalvia Vertebrate Amphibian Chordata Amphibia Invertebrate Arthropod Arthropoda Insecta Vertebrate Fish Chordata Actinopterygii Invertebrate Mollusk Mollusca Bivalvia Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Invertebrate Arthropod Arthropoda Insecta Vertebrate -

Heteroptera: Coreidae) En Perú
Ecología Aplicada, 14(1), 2015 Presentado: 25/08/2014 ISSN 1726-2216 Aceptado: 15/05/2015 Depósito legal 2002-5474 © Departamento Académico de Biología, Universidad Nacional Agraria La Molina, Lima – Perú. DISTRIBUCIÓN DE LAS ESPECIES DE OCHO GÉNEROS DE LA TRIBU COREINI (HETEROPTERA: COREIDAE) EN PERÚ DISTRIBUTION OF THE SPECIES OF EIGHT GENERA OF THE COREINI TRIBE (HETEROPTERA: COREIDAE) IN PERU Luis Cruces1 y Clorinda Vergara2 Resumen Coreini es la tribu de la Familia Coreidae que alberga el mayor número de especies en regiones tropicales, algunas de las cuales son citadas como plagas en diversos cultivos en el mundo. Para contribuir al conocimiento de la diversidad de coreidos en Perú, se elaboró un catálogo de las especies de Coreini. Se examinaron ejemplares pertenecientes a la colección del Museo de Entomología “Klaus Raven Büller” de la Universidad Nacional Agraria La Molina en Lima, Perú. La identificación se basó en claves taxonómicas y descripciones proporcionadas por Brailovsky (1985-2012). Se determinaron 23 especies correspondientes a ocho géneros: Acidomeria, Anasa, Althos, Catorhintha, Cebrenis, Sphictyrtus, Vazquezitocoris y Zicca. Se presenta la distribución de las especies de estos géneros conocidas para Perú, así como nuevos datos distribucionales. Las especies con distribución más amplia son Zicca signoreti, Z. rubricator rubricator, Z. impicta, y Anasa bellator, encontradas en por lo menos seis departamentos del Perú. Palabras clave: Acidomeria, Anasa, Althos, Catorhintha, Cebrenis, Sphictyrtus, Vazquezitocoris, Zicca, distribución, diversidad. Abstract Coreini is a tribe that belongs to the Coreidae family. It has the largest number of species in tropical regions, and some of them are cited as crop pests over the world.