Regulation of Urea Cycle Related Enzymes in Response to Freezing in the Wood Frog, Rana Sylvatica
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development of a System of High Ornithine and Citrulline Production by a Plant-Derived Lactic Acid Bacterium, Weissella Confusa K-28
Development of a system of high ornithine and citrulline production by a plant-derived lactic acid bacterium, Weissella confusa K-28 㸦᳜≀⏤᮶ங㓟⳦ :HLVVHOODFRQIXVD. ࠾ࡅࡿ ࢜ࣝࢽࢳࣥཬࡧࢩࢺࣝࣜࣥ㧗⏕⏘ࢩࢫࢸ࣒ࡢᵓ⠏㸧 Md Rakhimuzzaman Student ID: D164180 Supervisor: Prof. Masanori Sugiyama Department of Probiotic Science for Preventive Medicine Graduate School of Biomedical and Health Sciences Hiroshima University, Japan 2019 ABBREVIATIONS ADI arginine deiminase AUC area under curve BLAST basic local alignment search tool CK carbamate kinase dNTP deoxyribonucleotide triphosphate DDBJ DNA Data Bank of Japan EDTA ethylenediamine tetra-acetic acid EtOH ethanol EPS exopolysaccharide GABA gamma-aminobutyric acid GRAS generally recognized as safe HPLC high performance liquid chromatography LAB lactic acid bacteria Lb. Lactobacillus OD optical density OTC ornithine carbamoyltransferase PITC phenyl isothiocyanate TAE tris-acetate-ethylenediamine tetra-acetic acid TE tris-ethylenediamine tetra-acetic acid TEA triethylamine Tris tris(hydroxymethyl)aminomethane rDNA ribosomal deoxyribonucleic acid RT-PCR reverse transcription polymerase chain reaction CONTENTS INTRODUCTION 1-17 OBJECTIVES 18 MATERIALS AND METHODS 19-34 RESULTS 35-57 DISCUSSIONS 58-65 CONCLUSION 66-67 ACKNOWLEDGEMENTS 68 REFERENCES 69-84 INTRODUCTION Lactic acid bacteria (LAB) are an order of gram-positive, acid-tolerant, generally nonsporulating, non-respiring, either rod-shaped or spherical microorganisms. Since LAB lack the ability of synthesizing porphyrins (heme, and components of respiratory chains) (Pessione et al, 2010), the bacteria can not generate ATP without external heme supplementation (Pessione et al, 2010). The LAB strains can only obtain ATP by fermentation, usually they generate the ATP from some sugars. Because LAB do not use oxygen for energy production, the bacteria easily grow under anaerobic conditions, but they can also grow in presence of oxygen. -

The Internal Environment of Animals: 32 Organization and Regulation
CHAPTER The Internal Environment of Animals: 32 Organization and Regulation KEY CONCEPTS 32.1 Animal form and function are correlated at all levels of organization 32.2 The endocrine and nervous systems act individually and together in regulating animal physiology 32.3 Feedback control maintains the internal environment in many animals 32.4 A shared system mediates osmoregulation and excretion in many animals 32.5 The mammalian kidney’s ability to conserve water is a key terrestrial adaptation ▲ Figure 32.1 How do long legs help this scavenger survive in the scorching desert heat? Diverse Forms, Common Challenges Because form and function are correlated, examining anat- omy often provides clues to physiology—biological function. he desert ant (Cataglyphis) in Figure 32.1 scavenges In the case of the desert ant, researchers noted that its stilt-like insects that have succumbed to the daytime heat of the legs are disproportionately long, elevating the rest of the ant Sahara Desert. To gather corpses for feeding, the ant 4 mm above the sand. At this height, the ant’s body is exposed Tmust forage when surface temperatures on the sunbaked sand to a temperature 6°C lower than that at ground level. The ant’s exceed 60°C (140°F), well above the thermal limit for virtually long legs also facilitate rapid locomotion: Researchers have all animals. How does the desert ant survive these conditions? found that desert ants can run as fast as 1 m/sec, close to the To address this question, we need to consider the relationship top speed recorded for a running arthropod. -

Number 9 September 2019
VolumeVolume 23 1 -- NumberNumber 91 MaySeptember - September 2019 1997 Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL INIST-CNRS Scope The Atlas of Genetics and Cytogenetics in Oncology and Haematology is a peer reviewed on-line journal in open access, devoted to genes, cytogenetics, and clinical entities in cancer, and cancer-prone diseases. It is made for and by: clinicians and researchers in cytogenetics, molecular biology, oncology, haematology, and pathology. One main scope of the Atlas is to conjugate the scientific information provided by cytogenetics/molecular genetics to the clinical setting (diagnostics, prognostics and therapeutic design), another is to provide an encyclopedic knowledge in cancer genetics. The Atlas deals with cancer research and genomics. It is at the crossroads of research, virtual medical university (university and post-university e-learning), and telemedicine. It contributes to "meta-medicine", this mediation, using information technology, between the increasing amount of knowledge and the individual, having to use the information. Towards a personalized medicine of cancer. It presents structured review articles ("cards") on: 1- Genes, 2- Leukemias, 3- Solid tumors, 4- Cancer-prone diseases, and also 5- "Deep insights": more traditional review articles on the above subjects and on surrounding topics. It also present 6- Case reports in hematology and 7- Educational items in the various related topics for students in Medicine and in Sciences. The Atlas of Genetics and Cytogenetics -

Metabolic Reprogramming in Prostate Cancer
www.nature.com/bjc REVIEW ARTICLE Metabolic reprogramming in prostate cancer Fahim Ahmad 1,2, Murali Krishna Cherukuri2 and Peter L. Choyke1 Although low risk localised prostate cancer has an excellent prognosis owing to effective treatments, such as surgery, radiation, cryosurgery and hormone therapy, metastatic prostate cancer remains incurable. Existing therapeutic regimens prolong life; however, they are beset by problems of resistance, resulting in poor outcomes. Treatment resistance arises primarily from tumour heterogeneity, altered genetic signatures and metabolic reprogramming, all of which enable the tumour to serially adapt to drugs during the course of treatment. In this review, we focus on alterations in the metabolism of prostate cancer, including genetic signatures and molecular pathways associated with metabolic reprogramming. Advances in our understanding of prostate cancer metabolism might help to explain many of the adaptive responses that are induced by therapy, which might, in turn, lead to the attainment of more durable therapeutic responses. British Journal of Cancer https://doi.org/10.1038/s41416-021-01435-5 BACKGROUND Several tools have been designed to assess metabolism without Cellular metabolism involves complex biochemical processes disturbing the system. Advances in nuclear magnetic resonance through which specific nutrients are consumed. Carbohydrates, and mass spectroscopy have helped to quantify the carbon influx fatty acids and amino acids are the main source of nutrients for of the central metabolic pathways (e.g. TCA cycle, glycolysis and energy homeostasis and macromolecular synthesis. They are the pentose phosphate pathway) in mammalian systems, and this main constituent of core metabolic pathways that can be approach has been instrumental in demonstrating that the classified as anabolic, catabolic or waste producing (Fig. -

A NOVEL FORMULATION of HERBS with POTENT ANTI-AGEING ACTIVITY Mohit M
European Journal of Bioinformatics, Vol. 2:1-5, 2014 A NOVEL FORMULATION OF HERBS WITH POTENT ANTI-AGEING ACTIVITY Mohit M. Jaina, Nirmala Kumaria, Geeta Raib* aNEISS Wellness India Limited, Mumbai, India. bDepartment of Molecular and Human Genetics, Banaras Hindu University, Varanasi 221005, India. ABSTRACT Ageing is characterized by a progressive deterioration of physiological functions and metabolic processes. Sirtuin (SIRT1) is a NAD+-dependent protein deacetylase which is a human ortholog of the yeast SIR2 protein. The activation of SIRr2 affects wide variety of cellular processes ranging from metabolism, cell cycle, growth and differentiation, inflammation, senescence, apoptosis, stress response and ageing. There are natural anti- ageing drugs in the market like Resveraterol but some studies suggest that due to its interaction with contraceptive pills it should not be consumed by women taking them. Although, a diet rich in antioxidants seems to offer hope in delaying the onset of unhealthy disorders that accompany ageing, however no clinical treatment using natural herbs has yet been developed and natural anti-aging drugs are still unavailable. In the present study, we evaluated the binding of active ligands present in real grass with the SIRT1 and SIRT5 receptor and compared their binding energy and affinity with other reference anti-ageing drugs present in the market. A high throughput screening comprising of molecular docking, absorption, distribution, metabolism, excretion and toxicity predictions, logP values and percentage of human oral absorption value led to the identification of three potential compounds present in ‘Live Green Real Grass’ which could be considered for anti–ageing activity with better binding affinities than the reference drugs used. -

(07) 1. Salts and Minerals Are Conducted in Plants by Phloem Vascular Tissue 2
Answer Key Paper Code: 55857 Dated: 1.4.2019 Q. 1. A) Fill in the blanks: (07) 1. Salts and minerals are conducted in plants by phloem vascular tissue 2. Tendon connects _muscle__ to bones in humans 3. Requirement of Ca for the growth of plant is _macro type of nutrient 4. The part of the neuron cell containing nucleus is called as__cyton__. 5. Lymphatic circulatory system in animals helps in __immune response 6. The process of loss of salts happens through the __guttation of the plant 7. Heart is an involuntary muscle of the animal tissues. B) Match the columns: (07) Column A Column B a) Xylem i) Invertebrates (c) b) Phloem ii) Na+ ions (e) c) Hydrostatic Skeleton iii) Water Transport (a) d) Pectoral Girdle iv) Earthworm (f) e) Proton Pump v) Axial Skeleton (d) f) Circular Muscles vi) Stomata (g) g) Transpiration vii) Organic Solutes (b) C) Define / Explain the following terms: (06) 1. Excretion: Excretion is the removal of metabolic waste from the body. In animal kingdom of metabolism there are several strategies used by organism to eliminate the waste product of metabolism. 2. Alcoholic fermentation: is the anaerobic pathway carried out by yeasts in which simple sugars are converted to ethanol and carbon dioxide. 3. Partial Pressure:In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture. -

Effects of Fish Size and Feeding Frequency on Metabolism of Juvenile Walleye
Effects of fish size and feeding frequency on metabolism of juvenile walleye by Timothy Keith Yager A Thesis Submitted to the Graduate Faculty in Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE Department:· Animal Ecology Interdepartmental Major: Water Resources Signatures have been redacted for privacy Iowa State University Ames, Iowa 1991 ii TABLE OF CONTENTS Page GENERAL INTRODUCTION 1 Oxygen Consumption and Fish Respiration 2 Ammonia Excretion 4 Aeration and Ammonia Treatment Options 5 Factors Affecting Oxygen Consumption and Ammonia Excretion 7 Objectives 7 Explanation of Thesis Format 9 SECTION I. EFFECTS OF SIZE AND FEEDING FREQUENCY ON METABOLISM OF JUVENILE WALLEYE 10 ABSTRACT 11 INTRODUCTION 12 METHODS 15 culture Conditions 15 Feeding 17 Daily Monitoring of Ammonia-N (TAN) 19 24-hour Feeding Trials 20 Fish Lengths and Weights 22 Biomass Estimates 24 Mass-specific Metabolic Rates 24 statistical Analysis 28 iii RESULTS 31 Effects of Size 31 Oxygen consumption 31 Ammonia excretion 37 Daily ammonia excretion 46 Effects of Feeding Schedule 46 Oxygen consumption 46 Ammonia excretion 51 DISCUSSION 52 Effects of Size 52 Effects of Feeding Frequency 53 ACKNOWLEDGMENTS 55 REFERENCES 56 SECTION II. PATTERNS OF SPECIFIC DYNAMIC ACTION IN JUVENILE WALLEYE UNDER FOUR DIFFERENT FEEDING FREQUENCIES 59 ABSTRACT 60 INTRODUCTION 61 METHODS 64 Culture Conditions 64 Feeding 66 24-hour Feeding Trials 68 Biomass Estimates 70 Mass-specific Metabolic Rates 70 statistical Analysis 72 iv RESULTS 75 Patterns of SDA 75 One feeding 75 Two feedings 75 Three feedings 82 Multiple feedings 82 ANOVA on 3-h Mean Metabolic Rates 89 One feeding 89 Two feedings 89 Three feedings 94 Multiple feedings 94 DISCUSSION 98 ACKNOWLEDGMENTS 100 REFERENCES 101 SUMMARY AND DISCUSSION 104 LITERATURE CITED 106 ACKNOWLEDGMENTS 113 GENERAL INTRODUCTION Because the walleye (stizostedion vi~reum) is a highly prized sport and food fish, interest in the commercial production of walleye as a food fish has grown. -

Amino Acid Degradation; Urea Cycle
Amino acid degradation; urea cycle Kristina Mlinac Jerković [email protected] In animals, amino acids undergo oxidative degradation in three different metabolic circumstances: 1. During the normal synthesis and degradation of cellular proteins, some amino acids that are released from protein breakdown and are not needed for new protein synthesis undergo oxidative degradation. 2. When a diet is rich in protein and the ingested amino acids exceed the body’s needs for protein synthesis, the surplus is catabolized; amino acids cannot be stored. 3. During starvation or in uncontrolled diabetes mellitus, when carbohydrates are either unavailable or not properly utilized, cellular proteins are used as fuel. Metabolic fates of amino groups - The metabolism of nitrogen-containing molecules such as proteins and nucleic acids differs significantly from that of carbohydrates and lipids. - Whereas the latter molecules can be stored and mobilized as needed for biosynthetic reactions or for energy generation, there is no nitrogen-storing molecule (one exception to this rule is storage protein in seeds). - Organisms must constantly replenish their supply of usable nitrogen to replace organic nitrogen that is lost in catabolism. For example, animals must have a steady supply of amino acids in their diets to replace the nitrogen excreted as urea, uric acid, and other nitrogenous waste products. - Since excess dietary amino acids are neither stored for future use nor excreted, they are converted to common metabolic intermediates such as pyruvate, oxaloacetate, acetyl-CoA, and α-keto-glutarate. - Consequently, amino acids are also precursors of glucose, fatty acids, and ketone bodies and are therefore metabolic fuels. -

Protein Metabolism and It's Disorders
Protein Metabolism and it’s Disorders (Course : M.Sc. Zoology) Course Code: ZOOL 4008 (Biochemistry and Metabolism), Semester –II Dr. Shyam Babu Prasad Assistant Professor Department of Zoology Mahatma Gandhi Central University (MGCU), Motihari-845401 (Bihar) Email: [email protected] Important facts of the Protein Metabolism •Proteins metabolism referred as synthesis of protein from Amino acids (Anabolism) and breakdown of proteins (Catabolism). •In the Unit II, we discussed the synthesis (Anabolism) of various form of the proteins from different amino acids. • Here we mainly focused on the Catabolism of the protein, which is integral part of metabolism of the nitrogen-containing molecules. • Nitrogen enters in to the body in various form of compounds that is present in food, the most important is amino acids (as dietary food). • During Protein/amino acid metabolism (Catabolism), Nitrogen leaves the body as urea, ammonia, and in form of other derivatives. • The Catabolism of the protein depends on the amino acid pool and protein turnover (the rate of protein synthesis and degradation is equal). • The amino acids present in through out of the body (cells, blood, and the extracellular fluids etc) is called amino acid pools. It is maintained by following factors as represented in below diagram: Body protein Dietary degradation Protein 400gm/day 600gm/day Supplied Amino acid pool Depleted Synthesis of Remaining Synthesis of Glucose, Synthesis of amino acids Nitrogen containing Glycogen, the Body burned as compound like Ketone Proteins -
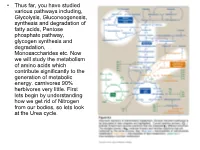
Transaminations and Urea Cycle
• Thus far, you have studied various pathways including, Glycolysis, Gluconeogenesis, synthesis and degradation of fatty acids, Pentose phosphate pathway, glycogen synthesis and degradation, Monosaccharides etc. Now we will study the metabolism of amino acids which contribute significantly to the generation of metabolic energy. carnivores 90% herbivores very little. First lets begin by understanding how we get rid of Nitrogen from our bodies, so lets look at the Urea cycle. 1 α Amino group keeps amino acids safe from oxidative breakdown. Removal of this group is essential for energy production it is an obligatory step for catabolism. Nitrogen can then be incorporated or excreted.This is done through transamination or oxidative deamination, provide amonia and aspartate sources for the Urea cycle. Transamination is funneled through glutamate. This is done through the transfer of the α amino group to α ketoglutarate. Product α-keto acid and glutamate. Alanine to pyruvate and aspartate to oxaloacetate all amino acids except lysine and threonina (lose the α amino through deamination. Glutamate dehydrogenase the oxidative deamination Glutamate only amino acid that undergoes rapid oxidative deamination (glutamate dehydrogenase NH3 + NADH), where amino groups are released as ammonia. After a meal rich in protein the levels of glutamate in liver are increased favoring amino acid degradation and formation of ammonia. Activators of this enzyme are ADP and GDP while ATP and GTP are inhibitors. This reaction occurs in the mitochondria. Ammonia can be transported to the liver from other tissues through two mechanisms that reduce the toxicity of ammonia. One through Glutamine (most tissues through, glutamine synthetase). In the liver the glutamine is converted back to glutamate (Fig. -

Cps1) for Studying This Enzyme’S
PhD THESIS USING RECOMBINANT HUMAN CARBAMOYL PHOSPHATE SYNTHETASE 1 (CPS1) FOR STUDYING THIS ENZYME’S FUNCTION, REGULATION, PATHOLOGY AND STRUCTURE Author: CARMEN DÍEZ FERNÁNDEZ Directors: Drs. Vicente Rubio Zamora y Javier Cervera Miralles May 2015 INSTITUTO DE BIOMEDICINA DE VALENCIA (IBV) Vicente Rubio Zamora, Doctor en Medicina, Profesor de Investigación del Consejo Superior de Investigaciones Científicas (CSIC) y Profesor Titular de Bioquímica (en Excedencia) de la Universidad de Valencia, y Javier Cervera Miralles, Doctor en Biología, Investigador jubilado del Centro de Investigación Príncipe Felipe de Valencia, de la Fundación Valenciana de Investigaciones Biomédicas de Valencia, CERTIFICAN: Que Dª, Carmen Díez Fernández, Ingeniera Agrónoma por la Universidad Politécnica de Valencia, con DNI 29208872E, ha realizado bajo su dirección el trabajo de Tesis Doctoral que lleva por título (en lengua inglesa): “Using recombinant human carbamoyl phosphate synthetase 1 (CPS1) for studying this enzyme’s function, regulation, pathology and structure”. Que están de acuerdo con los contenidos del trabajo, realizado en la modalidad de acopio de publicaciones. Que entienden que el trabajo reúne los requisitos para ser susceptible de defensa ante el Tribunal apropiado, por incluir dos publicaciones ya aparecidas y una sometida a evaluación, en revistas internacionales de amplia difusión y en las que la doctoranda es primera autora; así como porque el contenido de las otras secciones de dicho trabajo de tesis reflejan adecuadamente el estado del conocimiento y discuten apropiadamente el contenido y las implicaciones del trabajo realizado; porque las conclusiones recogen un avance suficiente en el campo objeto de estudio como para hacer a la candidata merecedora del doctorado; y, finalmente, porque les consta que ninguno de los coautores de dichas publicaciones, todos ellos ya doctores, han utilizado ni utilizarán las mismas para confeccionar otro trabajo de tesis doctoral. -

Granulosa-Lutein Cell Sirtuin Gene Expression Profiles Differ Between Normal Donors and Infertile Women
International Journal of Molecular Sciences Article Granulosa-Lutein Cell Sirtuin Gene Expression Profiles Differ between Normal Donors and Infertile Women Rebeca González-Fernández 1, Rita Martín-Ramírez 1, Deborah Rotoli 1,2, Jairo Hernández 3, Frederick Naftolin 4, Pablo Martín-Vasallo 1 , Angela Palumbo 3,4 and Julio Ávila 1,* 1 Laboratorio de Biología del Desarrollo, UD de Bioquímica y Biología Molecular and Centro de Investigaciones Biomédicas de Canarias (CIBICAN), Universidad de La Laguna, Av. Astrofísico Sánchez s/n, 38206 La Laguna, Tenerife, Spain; [email protected] (R.G.-F.); [email protected] (R.M.-R.); [email protected] (D.R.); [email protected] (P.M.-V.) 2 Institute of Endocrinology and Experimental Oncology (IEOS), CNR-National Research Council, 80131 Naples, Italy 3 Centro de Asistencia a la Reproducción Humana de Canarias, 38202 La Laguna, Tenerife, Spain; jairoh@fivap.com (J.H.); apalumbo@fivap.com (A.P.) 4 Department of Obstetrics and Gynecology, New York University, New York, NY 10016, USA; [email protected] * Correspondence: [email protected] Received: 13 November 2019; Accepted: 29 December 2019; Published: 31 December 2019 Abstract: Sirtuins are a family of deacetylases that modify structural proteins, metabolic enzymes, and histones to change cellular protein localization and function. In mammals, there are seven sirtuins involved in processes like oxidative stress or metabolic homeostasis associated with aging, degeneration or cancer. We studied gene expression of sirtuins by qRT-PCR in human mural granulosa-lutein cells (hGL) from IVF patients in different infertility diagnostic groups and in oocyte donors (OD; control group). Study 1: sirtuins genes’ expression levels and correlations with age and IVF parameters in women with no ovarian factor.