OMENTAL TRANSPLANTATION and CELL CULTURE. By: ROSENDO
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Sa G Es 2 0 0 6 Sages Lunches
TABLE OF CONTENTS 1 SAGES Corporate Supporters S 2 Hotel Contact Information THANKS TO OUR 2 General Information about the Meeting A 2 Registration Hours & Information CORPORATE SUPPORTERS: 2 Exhibits and Exhibit Only Registration G 5 SAGES Meeting Leaders PLATINUM LEVEL DONORS 7 SAGES Accreditation & CME Worksheet AUTOSUTURE & VALLEYLAB – E 8 Forde Tribute Dinner DIVISIONS OF TYCO HEALTHCARE 8 Hilton Anatole Floor Plan S 9 SAGES Schedule at a Glance ETHICON ENDO-SURGERY, INC. SAGES 2006 Postgraduate Courses 15 Bariatric Postgraduate Course KARL STORZ ENDOSCOPY-AMERICA, INC. 2 16 Joint SAGES-MIRA Symposium–Robotics 0 35 Colon Postgraduate Course OLYMPUS AMERICA 55 SAGES Allied Health Professionals Course GOLD LEVEL DONORS 0 SAGES 2006 Hands-On Courses 12 Joint IPEG/SAGES Pediatric Fellows Inamed Health 6 Advanced Techniques Hands-On Course Stryker Endoscopy 20 Surgeon in the Digital Age 27 Advanced Skills & Laparoscopic Techniques SILVER LEVEL DONORS Hands-On Course Boston Scientific Endoscopy 28 SAGES/SLS Simulator Hands-on Course Davol, Inc. 31 SAGES Endoluminal Surgery Hands-on Course General Surgery News 18 Joint SAGES/ACS Sessions Gore & Associates, Inc. 18 Inflammatory Bowel Disease 18 The Changing Face of Surgical Education BRONZE LEVEL DONORS 20 Ethicon Patient Safety Lunch Adolor Corporation and GlaxoSmithKline 22 International Video Session: Teleconferenced to Asia Aesculap 23 SAGES Technology Pavillion B-K Medical Systems 27 SAGES/IPEG Combined Video Breakfast Session Cook Surgical 32 SAGES/Fellowship Council Lunch 37 SAGES Hernia Symposium Medtronic 37 SAGES Bariatric Symposium SurgRX 39 SAGES 2006 Scientific Session Synovis Surgical Innovations 41 SAGES Presidential Address Taut, Inc. 43 Gerald Marks Lecture Tissue Science Laboratories 53 Karl Storz Lecture 44 SAGES/IPEG Panel, SAGES/ASGE Panel, Hernia Panel SAGES recognizes TATRC as a Meeting Supporter. -

Large Animal Surgical Procedures As-Of December 1, 2020 Abdominal
Large Animal Surgical Procedures as-of December 1, 2020 Core Curriculum Category Surgical Category Surgical Procedure Diaphragmatic herniorrhaphy Exploratory celiotomy - left flank Exploratory celiotomy - right flank Abdominal cavity/wall Exploratory celiotomy - ventral midline Exploratory celiotomy - ventral paramedian Exploratory laparotomy - death / euthanasia on table Peritoneal lavage via celiotomy Cecocolostomy Ileo-/Jejunocolostomy Cecum Jejunocecostomy Typhlectomy, partial Typhlotomy Abomasopexy, laparoscopic Abomasopexy, left flank Abdominal - LA Abomasopexy, paramedian Food animal GI: Abomasum Abomasotomy Omentopexy Pyloropexy, flank Reduction of volvulus Typhlectomy Food animal GI: Cecum Typhlotomy Food animal GI: Descending colon, Rectal prolapse, amputation/anastomosis rectum Rectal prolapse, submucosal reduction Food animal GI: Rumen Rumenotomy Decompression/emptying (no enterotomy) Food animal GI: Small intestine Enterotomy Reduction w/o resection (incarceration, volvulus, etc.) Resection/anastomosis Enterotomy Reduction of displacement Food animal GI: Spiral colon Reduction of volvulus Resection/anastomosis (inc. atresia coli) Side-side anastomosis, no resection Colopexy, hand-sutured Colopexy, laparoscopic Colostomy Large colon Enterotomy Reduction of displacement Reduction of volvulus Resection/anastomosis Biopsy Liver Cholelith removal Liver lobectomy Laceration repair Rectum Rectal prolapse repair Resection/anastomosis Enterotomy Impaction resolution via celiotomy Small colon Resection/anastomosis Taeniotomy Decompression/emptying -

Comparison of Laparoscopic-Guided Abomasopexy Versus Omentopexy Via Right Flank Laparotomy for the Treatment of Left Abomasal Displacement in Dairy Cows
Comparison of laparoscopic-guided abomasopexy versus omentopexy via right flank laparotomy for the treatment of left abomasal displacement in dairy cows Torsten Seeger, Dr Med Vet; Harald Kümper, Dr Med Vet; Klaus Failing, Dr Rer Nat; Klaus Doll, Dr Med Vet Habil mean lactation incidence is approximately 1% to 5% Objective—To compare results obtained by use of 3–7 laparoscopy-assisted abomasopexy versus omen- but is > 10% in some herds. Various surgical proce- topexy via right flank laparotomy for the treatment of dures have been used, all of which have specific advan- dairy cows with left displaced abomasum (LDA). tages and disadvantages. Open surgical techniques Animals—120 dairy cows with an LDA. include abomasopexy via the ventral paramedian approach8; laparotomy via the left paralumbar fossa for Procedure—In a prospective clinical trial, cows were omentopexy9 and abomasopexy,10 respectively; and randomly allocated to the abomasopexy group omentopexy via laparotomy in the right paralumbar (laparoscopy-assisted abomasopexy) or to the control 11 group (omentopexy via right flank). Data were fossa. Omentopexy via laparotomy in the right obtained during the first 5 days after surgery and 6 paralumbar fossa is considered the standard procedure weeks and 6 months after surgery. for the treatment of cattle with an LDA in Germany. Results—59 of 60 cows in the abomasopexy group Because of financial and time constraints, percuta- and all 60 cows in the control group were treated suc- neous fixation techniques, such as the blind-tack cessfully. Median duration was shorter for the laparo- suture procedure12 or toggle-pin method,13 have scopic procedure (27.5 minutes), compared with that become more commonly used by practitioners, even for the control group (38 minutes). -

Giant Peptic Ulcer Perforation- Omentopexy Versus Omental Plugging: a Comparative Study
International Surgery Journal Kumar R et al. Int Surg J. 2020 Mar;7(3):787-790 http://www.ijsurgery.com pISSN 2349-3305 | eISSN 2349-2902 DOI: http://dx.doi.org/10.18203/2349-2902.isj20200823 Original Research Article Giant peptic ulcer perforation- omentopexy versus omental plugging: a comparative study Rakesh Kumar1, Sneh Kiran2*, H. N. Singh Hariaudh1 1Department of General Surgery, NMCH, Patna, Bihar, India 2Department of Obstretics and Gynaecology, IGIMS, Patna, Bihar, India Received: 31 December 2019 Revised: 12 February 2020 Accepted: 13 February 2020 *Correspondence: Dr. Sneh Kiran, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Background: Giant peptic ulcer perforation is a life-threatening surgical emergency with high mortality rate. This study compares two different surgical techniques omentopexy and omental plugging for the treatment of giant peptic perforation. Methods: This study was a prospective study comparing the efficacy of omental plugging and omentopexy. The study was done at Emergency Department of General Surgery in Nalanda Medical College and Hospital, Patna over one-year period from October 2017 to September 2018. Patients were randomly allocated to two groups: one for omental plugging (cases) and other for omentopexy (controls). Results: A prospective non-randomized study of 12 patients with giant peptic perforation (≥2 cm in diameter) was carried out over a period of 24 months. -

Subject Index
Subject Index A – – complete 411 Abcarian, Herand 445 ––etiology 411 abdominal – – partial 411 – aortic aneuryms (AAA) 19, 46, 329 – – prevention 412 – aortic emergency 329 – – treatment 412 – – incision 331 – X-ray 21, 28, 33 – – infra-renal aortic control 332 abnormal – – operation 331 – gas pattern 33 – – preparation 331 – opacity 37 – – proximal control 332 abscess – – subdiaphragmatic aortic control 332 – complex form 382 – apoplexy 19, 88 – intra-abdominal 101, 211, 377 – bleeding – – conservative treatment 381 – – life-threatening 436 – perianal 258, 261 – closure 334, 337, 340 achalasia 113 – – high risk 340 acidosis – compartment syndrome 155, 319, 321 – lactic 56 – contamination 96, 97 – metabolic 56 – CT 372 acquired biliary strictures 173 – – reviewing 42 active bleeding 87 – decompression 326 acute – emergency – abdomen 10, 17 – – non-traumatic 441 – abdominal pain – exploration 87 – – in the fertile woman 276 – imaging 33 – anal fissure 263 – pain 19, 292 – appendicitis 21, 29, 44, 68, 210, 230, 245, – re-entry 85 281, 285, 293, – sepsis 96 – cholangitis 173 – trauma 9 – cholecystitis 21, 29, 163, 236, 281, 295 – – laparoscopy 442 – colitis 208 – ultrasound (US) 28 – diverticulitis 21, 229 – wall 53 – gastric mucosal lesion 125 ––defect 390 – gastroenteritis 37 ––hernia 191 – incarcerated full-thickness rectal – wall dehiscence 411 prolapse 261 458 Subject Index acute apoplexy 88 – mesenteric ischemia 19, 30, 197 appendectomy 250 – pancreatitis 19, 27, 95, 144, 175 appendiceal – perianal hematomy 263 – abscess 252 adhesion -

Asian Journal of Medical Research Original Article Abstract
Asian Journal of Medical Research Original Article Peptic perforation: epidemiology, etiology and management at tertiary care hospital in Gujarat Hiren Parmar 1, Asit Patel 1 1Department of Surgery,Smt N.H.L.Municipal Medical College, Ahmedabad,India. Abstract Objectives: The aims and objectives of the present study are as follows: (1) to assess value of clinical features and radiological investigations in cases of perforated peptic perforation (2) to study the relationship between tobacco, alcohol consumption and perforated peptic ulcer (3) to study the different operative methods (4) to study the histopathological diagnosis of ulcer margin (5) to study the mortality and post-operative complications (6) to assess complains in follow-up and study endoscopic findings. Methods: This was the prospective study consist of 50 cases of peptic perforation studied at general hospital during the period of 2003 to 2005. Results: 60% of patients were in age group of 21-50 years. 40% of patients were tobacco chewer. Abdominal pain was the commonest symptom of all patients. The surgical treatment in form of simple closure with omentopexy gives excellent results. Discussion: In this study the highest no of patients were in 5th decade of life. Male female ratio indicated male preponderance but decrease in the ratio as compare to previous study. Still plain x ray abdomen is the gold standard investigation in diagnosis. With better anaesthesia, higher antibiotics, and aggressive chest physiotherapy post-operative complications were reduced. Conclusion: The increasing incidence in female may be due to increasing tendency for women to take on the responsibilities and occupations traditionally associated with men. -
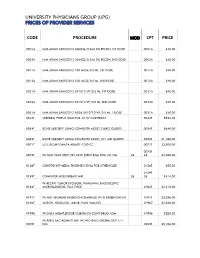
Code Procedure Cpt Price University Physicians Group
UNIVERSITY PHYSICIANS GROUP (UPG) PRICES OF PROVIDER SERVICES CODE PROCEDURE MOD CPT PRICE 0001A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 1ST DOSE 0001A $40.00 0002A IMM ADMN SARSCOV2 30MCG/0.3ML DIL RECON 2ND DOSE 0002A $40.00 0011A IMM ADMN SARSCOV2 100 MCG/0.5 ML 1ST DOSE 0011A $40.00 0012A IMM ADMN SARSCOV2 100 MCG/0.5 ML 2ND DOSE 0012A $40.00 0021A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 1ST DOSE 0021A $40.00 0022A IMM ADMN SARSCOV2 5X1010 VP/0.5 ML 2ND DOSE 0022A $40.00 0031A IMM ADMN SARSCOV2 AD26 5X10^10 VP/0.5 ML 1 DOSE 0031A $40.00 0042T CEREBRAL PERFUS ANALYSIS, CT W/CONTRAST 0042T $954.00 0054T BONE SURGERY USING COMPUTER ASSIST, FLURO GUIDED 0054T $640.00 0055T BONE SURGERY USING COMPUTER ASSIST, CT/ MRI GUIDED 0055T $1,188.00 0071T U/S LEIOMYOMATA ABLATE <200 CC 0071T $2,500.00 0075T 0075T PR TCAT PLMT XTRC VRT CRTD STENT RS&I PRQ 1ST VSL 26 26 $2,208.00 0126T CAROTID INT-MEDIA THICKNESS EVAL FOR ATHERSCLER 0126T $55.00 0159T 0159T COMPUTER AIDED BREAST MRI 26 26 $314.00 PR RECTAL TUMOR EXCISION, TRANSANAL ENDOSCOPIC 0184T MICROSURGICAL, FULL THICK 0184T $2,315.00 0191T PR ANT SEGMENT INSERTION DRAINAGE W/O RESERVOIR INT 0191T $2,396.00 01967 ANESTH, NEURAXIAL LABOR, PLAN VAG DEL 01967 $2,500.00 01996 PR DAILY MGMT,EPIDUR/SUBARACH CONT DRUG ADM 01996 $285.00 PR PERQ SAC AGMNTJ UNI W/WO BALO/MCHNL DEV 1/> 0200T NDL 0200T $5,106.00 PR PERQ SAC AGMNTJ BI W/WO BALO/MCHNL DEV 2/> 0201T NDLS 0201T $9,446.00 PR INJECT PLATELET RICH PLASMA W/IMG 0232T HARVEST/PREPARATOIN 0232T $1,509.00 0234T PR TRANSLUMINAL PERIPHERAL ATHERECTOMY, RENAL -

Research Article the Role of Perioperative Endoscopic Retrograde Cholangiopancreatography and Biliary Drainage in Large Liver Hydatid Cysts
Hindawi Publishing Corporation e Scientific World Journal Volume 2014, Article ID 301891, 6 pages http://dx.doi.org/10.1155/2014/301891 Research Article The Role of Perioperative Endoscopic Retrograde Cholangiopancreatography and Biliary Drainage in Large Liver Hydatid Cysts A. Krasniqi,1,2 B. Bicaj,1,2 D. Limani,1,2 M. Maxhuni,1,2 A. Rrusta,2 F. Hoxha,1,2 A. Hamza,1 V. Zejnullahu,2 F. Sada,1 S. Hashani,1,2 R. Musa,2 and R. Latifi3 1 Faculty of Medicine, University of Prishtina, 10000 Prishtina, Kosovo 2 Department of Surgery, University Clinical Centre of Kosova, 10000 Prishtina, Kosovo 3 Department of Surgery, The University of Arizona, Tucson, AZ 87524, USA Correspondence should be addressed to R. Latifi; [email protected] Received 28 June 2014; Revised 15 September 2014; Accepted 16 September 2014; Published 9 November 2014 Academic Editor: Shahzad G. Raja Copyright © 2014 A. Krasniqi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. The best surgical technique for large liver hydatid cysts (LHCs) has not yet been agreed on. Objectives.Theobjectiveof this study was to examine the role of perioperative endoscopic retrograde cholangiopancreatography (ERCP) and biliary drainage in patients with large LHCs. Methods. A 20-year retrospective study of patients with LHCs treated surgically at the University Clinical CenterofKosovo(UCCK).Wedividedpatientsinto2groupsbasedontreatmentperiod:1981–1990(GroupI)and2001–2010(Group II). Demographic characteristics (sex, age), the surgical procedure performed, complications rate, and outcomes were compared. -

Final Addenda Fy 1999 Icd-9-Cm Volume 3, Procedures
FINAL ADDENDA FY 1999 ICD-9-CM VOLUME 3, PROCEDURES Tabular List 36 Operations of vessels of heart Add code also Code also any injection or infusion of platelet inhibitor (99.20) 36.04 Intracoronary artery thrombolytic infusion Revise exclusion term Excludes: infusion of platelet inhibitor (99.20) infusion of thrombolytic agent (99.10) New category 36.3 Other heart revascularization Delete inclusion term Abrasion of epicardium Delete inclusion term Cardio-omentopexy Delete inclusion term Intrapericardial poudrage Delete inclusion term Myocardial graft: Delete inclusion term mediastinal fat Delete inclusion term omentum Delete inclusion term pectoral muscles New code 36.31 Open chest transmyocardial revascularization New code 36.32 Other transmyocardial revascularization Percutaneous transmyocardial revascularization Thoracoscopic transmyocardial revascularization New code 36.39 Other heart revascularization Abrasion of epicardium Cardio-omentopexy Intrapericardial poudrage Myocardial graft: mediastinal fat omentum pectoral muscles 37 Other operations on heart and pericardium Add code also Code also any injection or infusion of platelet inhibitor (99.20) 37.32 Excision of aneurysm of heart Add inclusion term Repair of aneurysm of heart New code 37.67 Implantation of cardiomyostimulation system Note: Two-step open procedure consisting of transfer of one end of the latissimus dorsi muscle; wrapping it around the heart; rib resection; implantation of epicardial cardiac pacing leads into the right ventricle; tunneling and pocket creation for -

A Study of Penetrating Thoracic and Abdominal Injuries
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 14, Issue 8 Ver. II (Aug. 2015), PP 64-95 www.iosrjournals.org A Study of Penetrating Thoracic and Abdominal Injuries Dr. Hardik Dodia, Dr. Keval Sansiya 1(General Surgery department, B.J. Medical College/ Gujarat University,India) 21(General Surgery department, B.J. Medical College/ Gujarat University,India) Abstract :In view of increasing number of penetrating thoracic or abdominal or combined injuries, this study has been chosen with reference to the patients presenting at Civil Hospitals Ahmedabad, affiliated with B.J. Medical college.This is a study of 25 cases. Age/Sex Incidence, Common viscera involved depending on site involved, operative procedures to be carried out according to viscera involved, Complications related to procedure and injuries, common cause of death have been highlighted in this study. Keywords: Penetrating injuries to thorax, penetrating abdominal injuries, common cause of death in penetrating trauma, Complications related to penetrating trauma. 1. INTRODUCTION Trauma remains the most common cause of death for all individuals between the ages of 1 and 44 years and is the third most common cause of death regardless of age. Penetrating thoracic or abdominal or combined injuries are one of the common injuries caused by assault. These injuries are associated with high risk of life threatening intra abdominal or intra thoracic organ injury. Due to the inadequate treatment of the injuries, many of the cases are fatal. The knowledge in the management of Penetrating trauma is progressively increasing due to the in-patient data gathered from different parts of the world. -

Clinical Practice Guidelines for the Treatment of Chronic Radiation Proctitis Ian M
CLINICAL PRACTICE GUIDELINES The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Chronic Radiation Proctitis Ian M. Paquette, M.D.1 • Jon D. Vogel, M.D.2 • Maher A. Abbas, M.D.3 Daniel L. Feingold, M.D.4 • Scott R. Steele, M.D., M.B.A.5 On behalf of the Clinical Practice Guidelines Committee of The American Society of Colon and Rectal Surgeons 1 University of Cincinnati Medical Center, Cincinnati, Ohio 2 Anschutz Medical Campus, University of Colorado Denver, Denver, Colorado 3 Al Zahra Hospital, Dubai, United Arab Emirates 4 Columbia University Medical Center, New York, New York 5 Cleveland Clinic, Cleveland, Ohio The American Society of Colon and Rectal Surgeons opment of a chronic hemorrhagic radiation proctitis. (ASCRS) is dedicated to ensuring high-quality patient care Chronic hemorrhagic radiation proctitis is a syndrome by advancing the science, prevention, and management marked by hematochezia, mucus discharge, tenesmus, of disorders and diseases of the colon, rectum, and anus. and, often, fecal incontinence.1 The incidence of this The Clinical Practice Guidelines Committee is charged condition was previously reported to be as high as 30%2; with leading international efforts in defining quality care however, with recent advances in radiation techniques, for conditions related to the colon, rectum, and anus by the delivery of a more targeted external beam radiation to developing clinical practice guidelines based on the best tumors will hopefully minimize collateral toxicity. Cur- available evidence. These guidelines are inclusive, not pre- rent estimates are that ~1% to 5% of patients treated with scriptive, and are intended for the use of all practitioners, radiation for pelvic malignancy will experience chronic healthcare workers, and patients who desire information radiation proctitis.1 Because of the nature of the symp- about the management of the conditions addressed by the toms associated with this condition, colorectal surgeons topics covered in these guidelines. -

Omental Wrapping” Technique with Autologous Onlay Flap/Graft Ureteroplasty for the Management of Long Ureteral Strictures
2878 Original Article The application of the “omental wrapping” technique with autologous onlay flap/graft ureteroplasty for the management of long ureteral strictures Jie Wang1#, Baiyu Zhang2#, Jian Fan1#, Sida Cheng1, Shubo Fan1, Lu Yin1, Zhihua Li1, Hua Guan1, Kunlin Yang1, Xuesong Li1 1Department of Urology, Peking University First Hospital, Institute of Urology, Peking University, National Urological Cancer Center, Beijing, China; 2Department of Urology, The First Affiliated Hospital of Kunming Medical University, Kunming, China Contributions: (I) Conception and design: J Wang, B Zhang; (II) Administrative support: X Li; (III) Provision of study materials or patients: X Li, K Yang; (IV) Collection and assembly of data: S Fan, L Yin, Z Li, H Guan; (V) Data analysis and interpretation: J Fan, S Cheng; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. #These authors contributed equally to this work. Correspondence to: Kunlin Yang; Xuesong Li. Department of Urology, Peking University First Hospital, Institute of Urology, Peking University, National Urological Cancer Center, No. 8 Xishiku St, Xicheng District, Beijing 100034, China. Email: [email protected]; [email protected]. Background: To present our experience with the “omental wrapping” technique in laparoscopic and robotic ureteroplasty using onlay flaps or grafts for the management of long proximal or middle ureteral strictures. Methods: This is a retrospective review of 25 patients with long proximal or middle ureteral strictures who underwent laparoscopic and robotic onlay flaps or grafts ureteroplasty using an omental flap to reinforce an anastomosis site between August 2018 and November 2019. Perioperative and follow-up data were collected. Results: Sixteen laparoscopic procedures and nine robotic procedures were performed successfully.