Cellular Immunology Sialoadhesin Unmarked
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse
Welcome to More Choice CD Marker Handbook For more information, please visit: Human bdbiosciences.com/eu/go/humancdmarkers Mouse bdbiosciences.com/eu/go/mousecdmarkers Human and Mouse CD Marker Handbook Human and Mouse CD Marker Key Markers - Human Key Markers - Mouse CD3 CD3 CD (cluster of differentiation) molecules are cell surface markers T Cell CD4 CD4 useful for the identification and characterization of leukocytes. The CD CD8 CD8 nomenclature was developed and is maintained through the HLDA (Human Leukocyte Differentiation Antigens) workshop started in 1982. CD45R/B220 CD19 CD19 The goal is to provide standardization of monoclonal antibodies to B Cell CD20 CD22 (B cell activation marker) human antigens across laboratories. To characterize or “workshop” the antibodies, multiple laboratories carry out blind analyses of antibodies. These results independently validate antibody specificity. CD11c CD11c Dendritic Cell CD123 CD123 While the CD nomenclature has been developed for use with human antigens, it is applied to corresponding mouse antigens as well as antigens from other species. However, the mouse and other species NK Cell CD56 CD335 (NKp46) antibodies are not tested by HLDA. Human CD markers were reviewed by the HLDA. New CD markers Stem Cell/ CD34 CD34 were established at the HLDA9 meeting held in Barcelona in 2010. For Precursor hematopoetic stem cell only hematopoetic stem cell only additional information and CD markers please visit www.hcdm.org. Macrophage/ CD14 CD11b/ Mac-1 Monocyte CD33 Ly-71 (F4/80) CD66b Granulocyte CD66b Gr-1/Ly6G Ly6C CD41 CD41 CD61 (Integrin b3) CD61 Platelet CD9 CD62 CD62P (activated platelets) CD235a CD235a Erythrocyte Ter-119 CD146 MECA-32 CD106 CD146 Endothelial Cell CD31 CD62E (activated endothelial cells) Epithelial Cell CD236 CD326 (EPCAM1) For Research Use Only. -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Functions of CD169 Positive Macrophages in Human Diseases (Review)
BIOMEDICAL REPORTS 14: 26, 2021 Functions of CD169 positive macrophages in human diseases (Review) YU LIU1, YUAN XIA2 and CHUN‑HONG QIU1 1Department of Cell Biology, School of Basic Medical Science, Cheeloo College of Medicine, Shandong University; 2Department of Hematology, Qilu Hospital of Shandong University, Jinan, Shandong 250012, P.R. China Received August 6, 2020; Accepted November 26, 2020 DOI: 10.3892/br.2020.1402 Abstract. CD169+ macrophages are a unique type of macro‑ 1. Introduction phage subset that differ from M1 and M2 macrophages. CD169+ macrophages are present in multiple tissues and Macrophages are distributed throughout the body in various organs throughout the body and are primarily expressed in tissues and organs and show a high degree of heterogeneity secondary lymphoid organs. These cells are primarily divided and diversity (1). Several specific markers expressed on across three locations in secondary lymphoid organs: The macrophage surfaces have been used to identify different metallophilic marginal zone of the spleen, the subcapsular subsets, such as F4/80, CD68, SRA‑1 and CD169 (2). CD169+ sinus and the medulla of the lymph nodes. Due to their unique macrophages are a unique subset of macrophages distributed location distribution in vivo and the presence of the CD169 across multiple tissues and organs of the human body. The molecule on their surfaces, CD169+ macrophages are reported results in the NCBI database showed that the CD169 mole‑ to serve important roles in several processes, such as phagocy‑ cules were expressed in 27 different tissues of the human tosis, antigen presentation, immune tolerance, viral infection body, such as the spleen, lymph node, small intestine, liver, and inflammatory responses. -

Human Lectins, Their Carbohydrate Affinities and Where to Find Them
biomolecules Review Human Lectins, Their Carbohydrate Affinities and Where to Review HumanFind Them Lectins, Their Carbohydrate Affinities and Where to FindCláudia ThemD. Raposo 1,*, André B. Canelas 2 and M. Teresa Barros 1 1, 2 1 Cláudia D. Raposo * , Andr1 é LAQVB. Canelas‐Requimte,and Department M. Teresa of Chemistry, Barros NOVA School of Science and Technology, Universidade NOVA de Lisboa, 2829‐516 Caparica, Portugal; [email protected] 12 GlanbiaLAQV-Requimte,‐AgriChemWhey, Department Lisheen of Chemistry, Mine, Killoran, NOVA Moyne, School E41 of ScienceR622 Co. and Tipperary, Technology, Ireland; canelas‐ [email protected] NOVA de Lisboa, 2829-516 Caparica, Portugal; [email protected] 2* Correspondence:Glanbia-AgriChemWhey, [email protected]; Lisheen Mine, Tel.: Killoran, +351‐212948550 Moyne, E41 R622 Tipperary, Ireland; [email protected] * Correspondence: [email protected]; Tel.: +351-212948550 Abstract: Lectins are a class of proteins responsible for several biological roles such as cell‐cell in‐ Abstract:teractions,Lectins signaling are pathways, a class of and proteins several responsible innate immune for several responses biological against roles pathogens. such as Since cell-cell lec‐ interactions,tins are able signalingto bind to pathways, carbohydrates, and several they can innate be a immuneviable target responses for targeted against drug pathogens. delivery Since sys‐ lectinstems. In are fact, able several to bind lectins to carbohydrates, were approved they by canFood be and a viable Drug targetAdministration for targeted for drugthat purpose. delivery systems.Information In fact, about several specific lectins carbohydrate were approved recognition by Food by andlectin Drug receptors Administration was gathered for that herein, purpose. plus Informationthe specific organs about specific where those carbohydrate lectins can recognition be found by within lectin the receptors human was body. -

Development and Validation of a Protein-Based Risk Score for Cardiovascular Outcomes Among Patients with Stable Coronary Heart Disease
Supplementary Online Content Ganz P, Heidecker B, Hveem K, et al. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA. doi: 10.1001/jama.2016.5951 eTable 1. List of 1130 Proteins Measured by Somalogic’s Modified Aptamer-Based Proteomic Assay eTable 2. Coefficients for Weibull Recalibration Model Applied to 9-Protein Model eFigure 1. Median Protein Levels in Derivation and Validation Cohort eTable 3. Coefficients for the Recalibration Model Applied to Refit Framingham eFigure 2. Calibration Plots for the Refit Framingham Model eTable 4. List of 200 Proteins Associated With the Risk of MI, Stroke, Heart Failure, and Death eFigure 3. Hazard Ratios of Lasso Selected Proteins for Primary End Point of MI, Stroke, Heart Failure, and Death eFigure 4. 9-Protein Prognostic Model Hazard Ratios Adjusted for Framingham Variables eFigure 5. 9-Protein Risk Scores by Event Type This supplementary material has been provided by the authors to give readers additional information about their work. Downloaded From: https://jamanetwork.com/ on 10/02/2021 Supplemental Material Table of Contents 1 Study Design and Data Processing ......................................................................................................... 3 2 Table of 1130 Proteins Measured .......................................................................................................... 4 3 Variable Selection and Statistical Modeling ........................................................................................ -

Defining the Role of CD47 and Sirpα in Murine B Cell Homeostasis
Defining the role of CD47 and SIRPα in murine B cell homeostasis Shrikant Shantilal Kolan Department of Integrative Medical Biology Umeå 2015 Responsible publisher under swedish law: the Dean of the Medical Faculty This work is protected by the Swedish Copyright Legislation (Act 1960:729) ISBN: 978-91-7601-324-3 ISSN: 0346-6612 Elektronisk version tillgänglig på http://umu.diva-portal.org/ Tryck/Printed by: Print and Media Umeå, Sweden 2015 Dedicated to my Family Table of Contents TABLE OF CONTENTS .............................................................................................. I ABSTRACT ............................................................................................................. V ABBREVIATIONS .................................................................................................. VII LIST OF ORIGINAL PUBLICATIONS ......................................................................... IX BACKGROUND ....................................................................................................... 1 IMMUNITY, THE IMMUNE SYSTEM AND IMMUNE CELLS ....................................................... 1 LYMPHOID ORGANS ..................................................................................................... 2 Primary lymphoid organs ................................................................................... 2 Secondary lymphoid organs ............................................................................... 3 MATURE B CELL POPULATIONS ...................................................................................... -

Sialoadhesin Binds Preferentially to Cells of the Granulocytic Lineage
Sialoadhesin binds preferentially to cells of the granulocytic lineage. P R Crocker, … , S Gordon, S Kelm J Clin Invest. 1995;95(2):635-643. https://doi.org/10.1172/JCI117708. Research Article Sialoadhesin is a macrophage-restricted, sialic acid-dependent receptor of 185 kD that binds to the oligosaccharide sequence NeuAc alpha 2,3Gal on cell surface glycoconjugates. Recent cDNA cloning has shown that sialoadhesin is a new member of the immunoglobulin superfamily with sequence similarity to CD22, a sialic acid-dependent receptor of B lymphocytes. Sialoadhesin has been implicated in cellular interactions of stromal macrophages with developing myeloid cells. In this study, direct evidence for this interaction was obtained in cell-cell binding assays using both native and recombinant forms of the protein. In all assays, sialoadhesin exhibited specific, differential binding to various murine cell populations of hemopoietic origin. In rank order, sialoadhesin bound neutrophils > bone marrow cells = blood leukocytes > lymphocytes > thymocytes. Single-cell analyses confirmed that sialoadhesin selectively bound myeloid cells in complex cell mixtures obtained from the bone marrow and blood. In comparison, a recombinant Fc-chimeric form of murine CD22 showed high binding to B and T lymphocytes, but very low binding to immature and mature myeloid cells. These results are consistent with the notion that sialoadhesin in involved in interactions with granulocytes at different stages of their life histories. Find the latest version: https://jci.me/117708/pdf -

CD33/Siglec-3 Binding Specificity, Expression Pattern, And
MOLECULAR AND CELLULAR BIOLOGY, June 2003, p. 4199–4206 Vol. 23, No. 12 0270-7306/03/$08.00ϩ0 DOI: 10.1128/MCB.23.12.4199–4206.2003 Copyright © 2003, American Society for Microbiology. All Rights Reserved. CD33/Siglec-3 Binding Specificity, Expression Pattern, and Consequences of Gene Deletion in Mice Els C. M. Brinkman-Van der Linden,1† Takashi Angata,1 Shirley A. Reynolds,1,2‡ Leland D. Powell,1§ Stephen M. Hedrick,1,2 and Ajit Varki1* Glycobiology Research and Training Center, Departments of Medicine and Cellular and Molecular Medicine,1 and Molecular Biology Section, Division of Biology,2 University of California, San Diego, La Jolla, California 92093-0687 Received 9 December 2002/Returned for modification 4 February 2003/Accepted 26 March 2003 Mouse CD33/Siglec-3 (mCD33) is the apparent ortholog of human CD33/Siglec-3 (hCD33), a member of the Siglec (sialic acid-binding Ig superfamily lectin) family of sialic acid-recognizing cell-surface lectins. We examined the binding specificity and expression pattern of mCD33 and explored its functions by generating mice deficient in this molecule. Like hCD33, mCD33 is expressed on myeloid precursors in the bone marrow, albeit mostly in the more mature stages of the granulocytic lineage. Moreover, unlike hCD33, mCD33 in peripheral blood is primarily expressed on granulocytes. Also, unlike hCD33, mCD33 did not bind to ␣2-3- or ␣2-6-linked sialic acids on lactosamine units. Instead, it showed distinctive sialic acid-dependent binding only to the short O-linked glycans of certain mucins and weak binding to the sialyl-Tn epitope. Binding was enhanced by removal of 9-O-acetyl groups and attenuated by truncation of the glycerol-like side chain of sialic acids. -
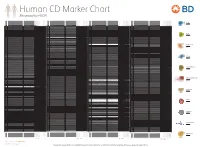
Reviewed by HLDA1
Human CD Marker Chart Reviewed by HLDA1 T Cell Key Markers CD3 CD4 CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD Alternative Name Ligands & Associated Molecules T Cell B Cell Dendritic Cell NK Cell Stem Cell/Precursor Macrophage/Monocyte Granulocyte Platelet Erythrocyte Endothelial Cell Epithelial Cell CD8 CD1a R4, T6, Leu6, HTA1 b-2-Microglobulin, CD74 + + + – + – – – CD74 DHLAG, HLADG, Ia-g, li, invariant chain HLA-DR, CD44 + + + + + + CD158g KIR2DS5 + + CD248 TEM1, Endosialin, CD164L1, MGC119478, MGC119479 Collagen I/IV Fibronectin + ST6GAL1, MGC48859, SIAT1, ST6GALL, ST6N, ST6 b-Galactosamide a-2,6-sialyl- CD1b R1, T6m Leu6 b-2-Microglobulin + + + – + – – – CD75 CD22 CD158h KIR2DS1, p50.1 HLA-C + + CD249 APA, gp160, EAP, ENPEP + + tranferase, Sialo-masked lactosamine, Carbohydrate of a2,6 sialyltransferase + – – + – – + – – CD1c M241, R7, T6, Leu6, BDCA1 b-2-Microglobulin + + + – + – – – CD75S a2,6 Sialylated lactosamine CD22 (proposed) + + – – + + – + + + CD158i KIR2DS4, p50.3 HLA-C + – + CD252 TNFSF4, -

CD System of Surface Molecules
THE CD SYSTEM OF LEUKOCYTE APPENDIX 4A SURFACE MOLECULES Monoclonal Antibodies to Human Cell Surface Antigens APPENDIX 4A Alice Beare,1 Hannes Stockinger,2 Heddy Zola,1 and Ian Nicholson1 1Women’s and Children’s Health Research Institute, Women’s and Children’s Hospital, Adelaide, Australia 2Institute of Immunology, University of Vienna, Vienna ABSTRACT Many of the leukocyte cell surface molecules are known by “CD” numbers. In this Appendix, a short introduction describes the history and the use of CD nomenclature and provides a few key references to enable access to the wider literature. This is followed by a table that lists all human molecules with approved CD names, tabulating alternative names, key structural features, cellular expression, major known functions, and usefulness of the molecules or antibodies against them in research or clinical applications. Curr. Protoc. Immunol. 80:A.4A.1-A.4A.73. C 2008 by John Wiley & Sons, Inc. Keywords: CD nomenclature r HLDA r HCDM r leukocyte marker r human leukocyte differentiation r antigens INTRODUCTION During the last 25 years, large numbers of monoclonal antibodies (MAbs) have been pro- duced that have facilitated the purification and functional characterization of a plethora of leukocyte surface molecules. The antibodies have been even more useful as markers for cell populations, allowing the counting, separation, and functional study of numer- ous subsets of cells of the immune system. A series of international workshops were instrumental in coordinating this development through multi-laboratory “blind” studies of thousands of antibodies. These HLDA (Human Leukocyte Differentiation Antigens) Workshops have, up until now, defined 500 different entities and assigned them cluster of differentiation (CD) designations. -

Regulation Pathway of Mesenchymal Stem Cell Immune Dendritic Cell
Downloaded from http://www.jimmunol.org/ by guest on September 26, 2021 is online at: average * The Journal of Immunology , 13 of which you can access for free at: 2010; 185:5102-5110; Prepublished online 1 from submission to initial decision 4 weeks from acceptance to publication October 2010; doi: 10.4049/jimmunol.1001332 http://www.jimmunol.org/content/185/9/5102 Inhibition of Immune Synapse by Altered Dendritic Cell Actin Distribution: A New Pathway of Mesenchymal Stem Cell Immune Regulation Alessandra Aldinucci, Lisa Rizzetto, Laura Pieri, Daniele Nosi, Paolo Romagnoli, Tiziana Biagioli, Benedetta Mazzanti, Riccardo Saccardi, Luca Beltrame, Luca Massacesi, Duccio Cavalieri and Clara Ballerini J Immunol cites 38 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription http://www.jimmunol.org/content/suppl/2010/10/01/jimmunol.100133 2.DC1 This article http://www.jimmunol.org/content/185/9/5102.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2010 by The American Association of -

Mannose Receptor Induces T-Cell Tolerance Via Inhibition of CD45 and Up-Regulation of CTLA-4
Mannose receptor induces T-cell tolerance via inhibition of CD45 and up-regulation of CTLA-4 Verena Schuettea,1, Maria Embgenbroicha,1, Thomas Ulasa, Meike Welzb, Jonas Schulte-Schreppinga, Astrid M. Draffehna, Thomas Quasta, Katharina Kocha, Melanie Nehringa, Jessica Königa, Annegret Zweynerta, Frederike L. Harmsa, Nancy Steinera, Andreas Limmerb, Irmgard Förstera, Friederike Berberich-Siebeltc, Percy A. Knolled, Dirk Wohlleberd, Waldemar Kolanusa, Marc Beyera, Joachim L. Schultzea, and Sven Burgdorfa,2 aLife and Medical Sciences Institute, University of Bonn, 53115 Bonn, Germany; bClinic for Orthopaedics and Trauma Surgery, University Hospital Bonn, 53127 Bonn, Germany; cInstitute of Pathology, University of Wurzburg, 97080 Wurzburg, Germany; and dInstitute of Molecular Immunology, Technische Universität München, 81675 Munich, Germany Edited by Emil R. Unanue, Washington University, St. Louis, MO, and approved July 26, 2016 (received for review April 12, 2016) The mannose receptor (MR) is an endocytic receptor involved in Results serum homeostasis and antigen presentation. Here, we identify + + The Presence of the MR on DCs Reduces Cytotoxic Activity of CD8 T the MR as a direct regulator of CD8 T-cell activity. We demonstrate Cells. To investigate whether the MR influences T-cell activation that MR expression on dendritic cells (DCs) impaired T-cell cytotox- by means distinct from antigen presentation, we made use of + icity in vitro and in vivo. This regulatory effect of the MR was me- Désiré T-cell receptor (DesTCR) transgenic CD8 T cells. Because diated by a direct interaction with CD45 on the T cell, inhibiting its these T cells recognize endogenous proteins (5), the presentation of phosphatase activity, which resulted in up-regulation of cytotoxic such antigens is independent of MR-mediated endocytosis.