Annual Report Contents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
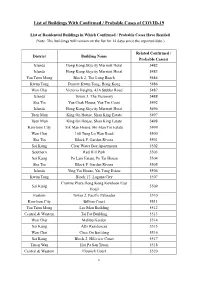
List of Buildings with Confirmed / Probable Cases of COVID-19
List of Buildings With Confirmed / Probable Cases of COVID-19 List of Residential Buildings in Which Confirmed / Probable Cases Have Resided (Note: The buildings will remain on the list for 14 days since the reported date.) Related Confirmed / District Building Name Probable Case(s) Islands Hong Kong Skycity Marriott Hotel 5482 Islands Hong Kong Skycity Marriott Hotel 5483 Yau Tsim Mong Block 2, The Long Beach 5484 Kwun Tong Dorsett Kwun Tong, Hong Kong 5486 Wan Chai Victoria Heights, 43A Stubbs Road 5487 Islands Tower 3, The Visionary 5488 Sha Tin Yue Chak House, Yue Tin Court 5492 Islands Hong Kong Skycity Marriott Hotel 5496 Tuen Mun King On House, Shan King Estate 5497 Tuen Mun King On House, Shan King Estate 5498 Kowloon City Sik Man House, Ho Man Tin Estate 5499 Wan Chai 168 Tung Lo Wan Road 5500 Sha Tin Block F, Garden Rivera 5501 Sai Kung Clear Water Bay Apartments 5502 Southern Red Hill Park 5503 Sai Kung Po Lam Estate, Po Tai House 5504 Sha Tin Block F, Garden Rivera 5505 Islands Ying Yat House, Yat Tung Estate 5506 Kwun Tong Block 17, Laguna City 5507 Crowne Plaza Hong Kong Kowloon East Sai Kung 5509 Hotel Eastern Tower 2, Pacific Palisades 5510 Kowloon City Billion Court 5511 Yau Tsim Mong Lee Man Building 5512 Central & Western Tai Fat Building 5513 Wan Chai Malibu Garden 5514 Sai Kung Alto Residences 5515 Wan Chai Chee On Building 5516 Sai Kung Block 2, Hillview Court 5517 Tsuen Wan Hoi Pa San Tsuen 5518 Central & Western Flourish Court 5520 1 Related Confirmed / District Building Name Probable Case(s) Wong Tai Sin Fu Tung House, Tung Tau Estate 5521 Yau Tsim Mong Tai Chuen Building, Cosmopolitan Estates 5523 Yau Tsim Mong Yan Hong Building 5524 Sha Tin Block 5, Royal Ascot 5525 Sha Tin Yiu Ping House, Yiu On Estate 5526 Sha Tin Block 5, Royal Ascot 5529 Wan Chai Block E, Beverly Hill 5530 Yau Tsim Mong Tower 1, The Harbourside 5531 Yuen Long Wah Choi House, Tin Wah Estate 5532 Yau Tsim Mong Lee Man Building 5533 Yau Tsim Mong Paradise Square 5534 Kowloon City Tower 3, K. -

Hong Kong Property: Not Crying Wolf
Equity Research 28 October 2013 Hong Kong Property Not crying wolf Barclays Capital Inc. and/or one of its affiliates does and seeks to do business with companies covered in its research reports. As a result, investors should be aware that the firm may have a conflict of interest that could affect the objectivity of this report. Investors should consider this report as only a single factor in making their investment decision. This research report has been prepared in whole or in part by research analysts based outside the US who are not registered/qualified as research analysts with FINRA. PLEASE SEE ANALYST(S) CERTIFICATION(S) AND IMPORTANT DISCLOSURES BEGINNING ON PAGE 132. Barclays | Hong Kong Property HONG KONG PROPERTY Not crying wolf Asia ex-Japan Real Estate First real downturn since 1998, home prices to fall 30% by end-2015E: We initiate Industry view: NEGATIVE coverage of the Hong Kong property sector with a negative view. We believe the property From Neutral market is about to enter its first real downturn since 1998 and we expect home prices to drop by at least 30% by the end of 2015. While we believe consensus also expects prices to decline, we think the magnitude of the fall is underestimated, as are the potential Paul Louie knock-on effects on commercial property. We expect a synchronised downturn, with +852 2903 4545 office prices falling by 20%, while retail properties escape with just zero growth through [email protected] end-2015E. Among the 14 stocks on which we initiate coverage, we rate eight UW, four Barclays Bank, Hong Kong EW and only two OW. -

1193Rd Minutes
Minutes of 1193rd Meeting of the Town Planning Board held on 17.1.2019 Present Permanent Secretary for Development Chairperson (Planning and Lands) Ms Bernadette H.H. Linn Professor S.C. Wong Vice-chairperson Mr Lincoln L.H. Huang Mr Sunny L.K. Ho Dr F.C. Chan Mr David Y.T. Lui Dr Frankie W.C. Yeung Mr Peter K.T. Yuen Mr Philip S.L. Kan Dr Lawrence W.C. Poon Mr Wilson Y.W. Fung Dr C.H. Hau Mr Alex T.H. Lai Professor T.S. Liu Ms Sandy H.Y. Wong Mr Franklin Yu - 2 - Mr Daniel K.S. Lau Ms Lilian S.K. Law Mr K.W. Leung Professor John C.Y. Ng Chief Traffic Engineer (Hong Kong) Transport Department Mr Eddie S.K. Leung Chief Engineer (Works) Home Affairs Department Mr Martin W.C. Kwan Deputy Director of Environmental Protection (1) Environmental Protection Department Mr. Elvis W.K. Au Assistant Director (Regional 1) Lands Department Mr. Simon S.W. Wang Director of Planning Mr Raymond K.W. Lee Deputy Director of Planning/District Secretary Ms Jacinta K.C. Woo Absent with Apologies Mr H.W. Cheung Mr Ivan C.S. Fu Mr Stephen H.B. Yau Mr K.K. Cheung Mr Thomas O.S. Ho Dr Lawrence K.C. Li Mr Stephen L.H. Liu Miss Winnie W.M. Ng Mr Stanley T.S. Choi - 3 - Mr L.T. Kwok Dr Jeanne C.Y. Ng Professor Jonathan W.C. Wong Mr Ricky W.Y. Yu In Attendance Assistant Director of Planning/Board Ms Fiona S.Y. -

ATTACHMENT 1 Barcode:3800584-02 C-570-107 INV - Investigation
ATTACHMENT 1 Barcode:3800584-02 C-570-107 INV - Investigation - Chinese Producers of Wooden Cabinets and Vanities Company Name Company Information Company Name: A Shipping A Shipping Street Address: Room 1102, No. 288 Building No 4., Wuhua Road, Hongkou City: Shanghai Company Name: AA Cabinetry AA Cabinetry Street Address: Fanzhong Road Minzhong Town City: Zhongshan Company Name: Achiever Import and Export Co., Ltd. Street Address: No. 103 Taihe Road Gaoming Achiever Import And Export Co., City: Foshan Ltd. Country: PRC Phone: 0757-88828138 Company Name: Adornus Cabinetry Street Address: No.1 Man Xing Road Adornus Cabinetry City: Manshan Town, Lingang District Country: PRC Company Name: Aershin Cabinet Street Address: No.88 Xingyuan Avenue City: Rugao Aershin Cabinet Province/State: Jiangsu Country: PRC Phone: 13801858741 Website: http://www.aershin.com/i14470-m28456.htmIS Company Name: Air Sea Transport Street Address: 10F No. 71, Sung Chiang Road Air Sea Transport City: Taipei Country: Taiwan Company Name: All Ways Forwarding (PRe) Co., Ltd. Street Address: No. 268 South Zhongshan Rd. All Ways Forwarding (China) Co., City: Huangpu Ltd. Zip Code: 200010 Country: PRC Company Name: All Ways Logistics International (Asia Pacific) LLC. Street Address: Room 1106, No. 969 South, Zhongshan Road All Ways Logisitcs Asia City: Shanghai Country: PRC Company Name: Allan Street Address: No.188, Fengtai Road City: Hefei Allan Province/State: Anhui Zip Code: 23041 Country: PRC Company Name: Alliance Asia Co Lim Street Address: 2176 Rm100710 F Ho King Ctr No 2 6 Fa Yuen Street Alliance Asia Co Li City: Mongkok Country: PRC Company Name: ALMI Shipping and Logistics Street Address: Room 601 No. -

Thailand) PCL, 45% (Of Paid-Up Capital)
Annual Report 2018 2018 ANNUAL REPORT PP PRIME PUBLIC COMPANY LIMITED PP PRIME PUBLIC COMPANY LIMITED Head Office: 69/5 Moo 5 Rama 2 Road, Bang Khan Taek, EMPOWERING Muangsamut Songkhram, Samut Songkhram 75000 FOR SUSTAINABILITY PP Prime Public Company Limited 1 3 4 7 8 18 34 37 43 44 45 64 75 95 96 99 100 111 112 118 2 Annual Report 2018 PP Prime Public Company Limited 3 4 Annual Report 2018 PP Prime Public Company Limited 5 Board of Directors 6 Annual Report 2018 Board of Directors Financial Information 2018 2017 2016 Sales (Baht) 2,121,449,514 1,875,523,686 2,240,181,809 Net Profit (Loss) (Baht) (110,084,444) (252,406,913) 232,314,148 Earning (Loss)per Share (Baht) (0.20) (0.45) 0.41 Total Asset (Baht) 3,339,876,952 3,987,770,023 3,569,307,914 Total Liabilities (Baht) 2,526,867,363 2,610,754,228 1,749,962,003 Shareholders’ Equity (Baht) 813,009,589 1,377,015,795 1,819,345,911 Book Value (Baht) 1.44 2.45 3.23 Return on Asset (%) (3.00) (6.68) 6.51 Return on Equity (%) (10.05) (15.79) 12.77 Debt to Shareholders (times) 3.11 1.90 0.96 Dividend Yield (%) 0.00 0.00 0.00 P/E Ratio (times) 0.00 0.00 0.00 PP Prime Public Company Limited 7 Policy and Business Overview ภาพรวมธุรกิจ Future TLUXE Food Farm The company focuses on developing the tiger shrimp for sale. During the past 30 years or so, the business in line with the full-cycle strategy i.e. -
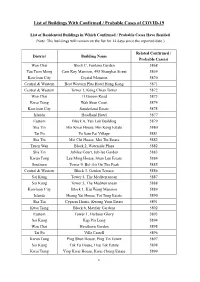
List of Buildings with Confirmed / Probable Cases of COVID-19
List of Buildings With Confirmed / Probable Cases of COVID-19 List of Residential Buildings in Which Confirmed / Probable Cases Have Resided (Note: The buildings will remain on the list for 14 days since the reported date.) Related Confirmed / District Building Name Probable Case(s) Wan Chai Block C, Fontana Garden 5868 Yau Tsim Mong Cam Key Mansion, 495 Shanghai Street 5869 Kowloon City Crystal Mansion 5870 Central & Western Best Western Plus Hotel Hong Kong 5871 Central & Western Tower 1, Kong Chian Tower 5872 Wan Chai 11 Broom Road 5873 Kwai Tsing Wah Shun Court 5874 Kowloon City Sunderland Estate 5875 Islands Headland Hotel 5877 Eastern Block A, Yen Lok Building 5879 Sha Tin Hin Kwai House, Hin Keng Estate 5880 Tai Po Po Sam Pai Village 5881 Sha Tin Mei Chi House, Mei Tin Estate 5882 Tsuen Wan Block 2, Waterside Plaza 5882 Sha Tin Jubilee Court, Jubilee Garden 5883 Kwun Tong Lee Ming House, Shun Lee Estate 5884 Southern Tower 9, Bel-Air On The Peak 5885 Central & Western Block 3, Garden Terrace 5886 Sai Kung Tower 5, The Mediterranean 5887 Sai Kung Tower 5, The Mediterranean 5888 Kowloon City Block 1, Kiu Wang Mansion 5889 Islands Heung Yat House, Yat Tung Estate 5890 Sha Tin Cypress House, Kwong Yuen Estate 5891 Kwai Tsing Block 6, Mayfair Gardens 5892 Eastern Tower 1, Harbour Glory 5893 Sai Kung Kap Pin Long 5894 Wan Chai Hawthorn Garden 5895 Tai Po Villa Castell 5896 Kwun Tong Ping Shun House, Ping Tin Estate 5897 Sai Kung Tak Fu House, Hau Tak Estate 5898 Kwai Tsing Ying Kwai House, Kwai Chung Estate 5899 1 Related Confirmed / -

COVID-19: China Medical Supply Chains and Broader Trade Issues
COVID-19: China Medical Supply Chains and Broader Trade Issues Updated December 23, 2020 Congressional Research Service https://crsreports.congress.gov R46304 SUMMARY R46304 COVID-19: China Medical Supply Chains and December 23, 2020 Broader Trade Issues Karen M. Sutter, The outbreak of Coronavirus Disease 2019 (COVID-19), first in China, and then Coordinator globally, including in the United States, has drawn attention to the ways in which the Specialist in Asian Trade U.S. economy depends on manufacturing and supply chains based in China. This report and Finance aims to assess current developments and identify immediate and longer range China trade issues for Congress. Andres B. Schwarzenberg Analyst in International An area of particular concern to Congress has been U.S. shortages in medical supplies— Trade and Finance including personal protective equipment (PPE) and pharmaceuticals—as the United States stepped up efforts to contain the COVID-19 pandemic with limited domestic Michael D. Sutherland stockpiles and insufficient U.S. industrial capacity. Because of China’s role as a global Analyst in International supplier of PPE, medical devices, antibiotics, and active pharmaceutical ingredients, Trade and Finance reduced exports from China led to shortages of critical medical supplies in the United States. Exacerbating the situation, in early February 2020, the Chinese government nationalized control of the production and distribution of medical supplies in China— directing all production for domestic use—and directed the bureaucracy and Chinese industry to secure supplies from the global market. Once past the initial peak of its COVID-19 outbreak, the Chinese government appears to have prioritized certain countries and selectively released some medical supplies for overseas delivery. -

Febrúar 2021
Hugverka tíðindi 38. árg | 2. tbl | 15. feb. 2021 Útgefandi: Hugverkastofan Ábyrgðarmaður: Borghildur Erlingsdóttir Afgreiðsla: Engjateigi 3, 105 Reykjavík Sími: 580 9400, Bréfasími: 580 9401 Afgreiðslutími: kl. 10-15 virka daga Heimasíða: www.hugverk.is Áskriftargjald: 4.300,- Verð í lausasölu: kr. 500,- eintakið Rafræn útgáfa: ISSN 1670-0104 Efnisyfirlit Vörumerki Einkaleyfi Skráð landsbundin vörumerki.......................................... 4 Veitt einkaleyfi (B)…………………………………………………….. 164 Birt landsbundin vörumerki………………………………………. 8 Evrópsk einkaleyfi í gildi á Íslandi (T3)............................ 165 Alþjóðlegar vörumerkjaskráningar.................................. 40 Breytt útgáfa evrópskra einkaleyfa í gildi á Íslandi eftir takmörkun (T4)……….………...……………………………………… 178 Breytingar í vörumerkjaskrá...……………………………….…… 58 Beiðni um endurveitingu…………………………………………… 179 Breyting skv. 54 gr. laga nr. 45/1997 um vörumerki…... 71 Umsóknir um viðbótarvernd (I1)……………….……………….. 180 Takmarkanir og viðbætur………………………………………….. 72 Veitt viðbótarvottorð (I2)…………………………………………… 181 Framsöl að hluta………………………………………………………. 73 Framlenging á viðbótarvottorði…………………………………. 182 Veðsetning vörumerkja…………………………………………….. 74 Breytingar í einkaleyfaskrá............................................... 183 Endurnýjuð vörumerki………………………………………………. 75 Leiðréttingar..................................................................... 185 Afmáð vörumerki……………………………………………………… 76 Andmæli………………………………………………………………….. 77 Úrskurðir í áfrýjunarmálum……………………………………….. 78 Hönnun -

Updated: Sep 14, 2020
Updated: Sep 14, 2020 ASHK members receive valuable offers and discounts from more than 30 selected retailers in Hong Kong. To enjoy these exclusive privileges, simply present your valid ASHK membership card at the participating outlets. Please check our website for the latest offers! Enjoy 10% discount on regular-priced items to 45R 45R has surpassed fashion and adhering to its belief in the beauty of nostalgia and quality craftsmanship. The timeless designs feel like an old favorite at the very first time it is worn and it is of such quality that 45R clothing can be passed on from generation to generation. Exquisite materials and craftsmanship conveys the luxurious-causal styling to daily life. Terms and conditions apply: - Promotion cannot be used in conjunction with any other promotions - Please present Asia Society Hong Kong Center Membership Card to shop staff before billing Shop Directory: - Valid until 31 December 2020 7, Star Street, Wan Chai, Tel: 2861 1145 - Benefits are subject to change without prior notice and Sidefame Shop 3083, IFC Mall, Tel: 2556 9745 Ltd reserves the right to decide on the eligibility of benefits Shop 201, The Lee Gardens One, Tel: 2504 2445 Shop 2515, Harbour City Tel: 2175 5545 Enjoy 15% discount at AMMO Residing at the Asia Society Hong Kong Center, AMMO is an Italian Japanese fusion restaurant inspired by the botanical surroundings. From today onwards, all ASHK members enjoy 15% off upon presentation of a valid Asia Society Hong Kong Center membership card when settling the bill. Hope you enjoy this new exclusive offers available to ASHK members only. -

Name of Buildings Awarded the Quality Water Supply Scheme for Buildings – Fresh Water (Plus) Certificate (As at 8 February 2018)
Name of Buildings awarded the Quality Water Supply Scheme for Buildings – Fresh Water (Plus) Certificate (as at 8 February 2018) Name of Building Type of Building District @Convoy Commercial/Industrial/Public Utilities Eastern 1 & 3 Ede Road Private/HOS Residential Kowloon City 1 Duddell Street Commercial/Industrial/Public Utilities Central & Western 100 QRC Commercial/Industrial/Public Utilities Central & Western 102 Austin Road Commercial/Industrial/Public Utilities Yau Tsim Mong 1063 King's Road Private/HOS Residential Eastern 11 MacDonnell Road Private/HOS Residential Central & Western 111 Lee Nam Road Commercial/Industrial/Public Utilities Southern 12 Shouson Hill Road Private/HOS Residential Central & Western 127 Repulse Bay Road Private/HOS Residential Southern 12W Commercial/Industrial/Public Utilities Tai Po 15 Homantin Hill Private/HOS Residential Yau Tsim Mong 15W Commercial/Industrial/Public Utilities Tai Po 168 Queen's Road Central Commercial/Industrial/Public Utilities Central & Western 16W Commercial/Industrial/Public Utilities Tai Po 17-19 Ashley Road Commercial/Industrial/Public Utilities Yau Tsim Mong 18 Farm Road (Shopping Arcade) Commercial/Industrial/Public Utilities Kowloon City 18 Upper East Private/HOS Residential Eastern 1881 Heritage Commercial/Industrial/Public Utilities Yau Tsim Mong 211 Johnston Road Commercial/Industrial/Public Utilities Wan Chai 225 Nathan Road Commercial/Industrial/Public Utilities Yau Tsim Mong Name of Buildings awarded the Quality Water Supply Scheme for Buildings – Fresh Water (Plus) -

Annual Report DBX ETF Trust
May 31, 2021 Annual Report DBX ETF Trust Xtrackers Harvest CSI 300 China A-Shares ETF (ASHR) Xtrackers Harvest CSI 500 China A-Shares Small Cap ETF (ASHS) Xtrackers MSCI All China Equity ETF (CN) Xtrackers MSCI China A Inclusion Equity ETF (ASHX) DBX ETF Trust Table of Contents Page Shareholder Letter ....................................................................... 1 Management’s Discussion of Fund Performance ............................................. 3 Performance Summary Xtrackers Harvest CSI 300 China A-Shares ETF ........................................... 6 Xtrackers Harvest CSI 500 China A-Shares Small Cap ETF .................................. 8 Xtrackers MSCI All China Equity ETF .................................................... 10 Xtrackers MSCI China A Inclusion Equity ETF ............................................ 12 Fees and Expenses ....................................................................... 14 Schedule of Investments Xtrackers Harvest CSI 300 China A-Shares ETF ........................................... 15 Xtrackers Harvest CSI 500 China A-Shares Small Cap ETF .................................. 20 Xtrackers MSCI All China Equity ETF .................................................... 28 Xtrackers MSCI China A Inclusion Equity ETF ............................................ 33 Statements of Assets and Liabilities ........................................................ 42 Statements of Operations ................................................................. 43 Statements of Changes in Net -

English Version
Indoor Air Quality Certificate Award Ceremony COS Centre 38/F and 39/F Offices (CIC Headquarters) Millennium City 6 Common Areas Wai Ming Block, Caritas Medical Centre Offices and Public Areas of Whole Building Premises Awarded with “Excellent Class” Certificate (Whole Building) COSCO Tower, Grand Millennium Plaza Public Areas of Whole Building Mira Place Tower A Public Areas of Whole Office Building Wharf T&T Centre 11/F Office (BOC Group Life Assurance Millennium City 5 BEA Tower D • PARK Baby Care Room and Feeding Room on Level 1 Mount One 3/F Function Room and 5/F Clubhouse Company Limited) Modern Terminals Limited - Administration Devon House Public Areas of Whole Building MTR Hung Hom Building Public Areas on G/F and 1/F Wharf T&T Centre Public Areas from 5/F to 17/F Building Dorset House Public Areas of Whole Building Nan Fung Tower Room 1201-1207 (Mandatory Provident Fund Wheelock House Office Floors from 3/F to 24/F Noble Hill Club House EcoPark Administration Building Offices, Reception, Visitor Centre and Seminar Schemes Authority) Wireless Centre Public Areas of Whole Building One Citygate Room Nina Tower Office Areas from 15/F to 38/F World Commerce Centre in Harbour City Public Areas from 5/F to 10/F One Exchange Square Edinburgh Tower Whole Office Building Ocean Centre in Harbour City Public Areas from 5/F to 17/F World Commerce Centre in Harbour City Public Areas from 11/F to 17/F One International Finance Centre Electric Centre 9/F Office Ocean Walk Baby Care Room World Finance Centre - North Tower in Harbour City Public Areas from 5/F to 17/F Sai Kung Outdoor Recreation Centre - Electric Tower Areas Equipped with MVAC System of The Office Tower, Convention Plaza 11/F & 36/F to 39/F (HKTDC) World Finance Centre - South Tower in Harbour City Public Areas from 5/F to 17/F Games Hall Whole Building Olympic House Public Areas of 1/F and 2/F World Tech Centre 16/F (Hong Yip Service Co.