Reproduction and Health in Holstein Warmblood Mares
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ESSA Courier No
ESSA courier No. 21 – April 2014 __________________________________________________________________________________ The President’s Editorial 2 __________________________________________________________________________________ New partner institution: the Portuguese National Stud ALTER REAL 3 __________________________________________________________________________________ This year MARBACH STATE STUD celebrates its 500th jubilee 4 __________________________________________________________________________________ REVIEW: ESSA presence at the Pferd Bodensee fair at Friedrichshafen, Germany 6 __________________________________________________________________________________ REVIEW: CAI3* KLADRUBY NAD LABEM with successful teams of the National Studs 7 KLADRUBY and TOPOL’ČIANKY __________________________________________________________________________________ Days of European Stud culture with General Assembly, Symposium and Marbach Classics, July 3rd to 5th at MARBACH STATE STUD 7 __________________________________________________________________________________ ESSA presents “Europa‐Quadrille” at the European horse fair Eurocheval at Offenburg, Germany, July 23rd to 27th 8 __________________________________________________________________________________ Register now for the excursion to YPÄJÄ Equine College and the King Trotting Race at Pori, Finland, August 1st to 4th 9 __________________________________________________________________________________ One year in Switzerland – internship report by Anja Schwarz 10 __________________________________________________________________________________ -

List of Horse Breeds 1 List of Horse Breeds
List of horse breeds 1 List of horse breeds This page is a list of horse and pony breeds, and also includes terms used to describe types of horse that are not breeds but are commonly mistaken for breeds. While there is no scientifically accepted definition of the term "breed,"[1] a breed is defined generally as having distinct true-breeding characteristics over a number of generations; its members may be called "purebred". In most cases, bloodlines of horse breeds are recorded with a breed registry. However, in horses, the concept is somewhat flexible, as open stud books are created for developing horse breeds that are not yet fully true-breeding. Registries also are considered the authority as to whether a given breed is listed as Light or saddle horse breeds a "horse" or a "pony". There are also a number of "color breed", sport horse, and gaited horse registries for horses with various phenotypes or other traits, which admit any animal fitting a given set of physical characteristics, even if there is little or no evidence of the trait being a true-breeding characteristic. Other recording entities or specialty organizations may recognize horses from multiple breeds, thus, for the purposes of this article, such animals are classified as a "type" rather than a "breed". The breeds and types listed here are those that already have a Wikipedia article. For a more extensive list, see the List of all horse breeds in DAD-IS. Heavy or draft horse breeds For additional information, see horse breed, horse breeding and the individual articles listed below. -

Ocala Jockey Club Breeding Info
Ocala Jockey Club HT Breeding Info Rider Last Name Rider First name Horse Sire Dam Dam Sire Breed Owner Breeder Division Sex Aden Nicole Truckee Bash Truckee xx Bashful Belle xx Stately Cielo xx Thoroughbred Woods Baughman Four Quarters Corp CCI4-S Gelding (CA) Aharoni Arielle Dutch Times Good Times Alino Queen Michelino Dutch Warmblood Christina Aharoni Lauren Efford CCI4-L Gelding Atkinson James Fleur de Lis Heartbreaker Matana U Hattrick Dutch Warmblood James Atkinson. Griendstveen HJJJ CCII3-L Gelding Jim Seilsopour Bouwmans Babbitt Charlotte 2 A.M. Sheraton Regina-K Ahorn Dutch Warmblood Charlotte Babbitt A. Lusseveld CCI3-L Gelding Baker Shanon Ballingowan Zeal Seabrook Shara Bride Clover Hill Irish Sport Horse Shannon Baker Tom Reilly CCI3-L Gelding Baugh Alexandera I Spye Harlequin Du Carel Lombardos Corner Lombardo Irish Sport Horse. Jesse Campbell Thomas Ryan CCI2-L Gelding Baugh Alexandra Mr. Candyman Canto 16 Montara Corofino I Holsteiner Altorac Farm Meerheimb H.W. CCI4-L Gelding Freiherr Von Beshear Emily El Mesano Mizzen Mast La Laja El Prado Thoroughbred Emily Beshear Helen K. Groves CCI2-L Gelding Revokable Trust Beshear Emily Deal With It Medaglia d’Oro Amada Unbridled Thoroughbred Emily Beshear CCI3-L Gelding Beshear Emily Templewood Horse Chestnut Missy Dear Deerhound Thoroughbred Catherine Birley Kenneth Tomlinson CCI2-L Gelding Black Maya Fe Chardonnay Clinton I Callina Compliment Hanoverian Light Speed CCI2-L Gelding Equestrian, LLC Black Maya Maks Mojo C Mighty Magic Winter Morning Ramiro’s Bube Hanoverian Laurie Cameron Laurie Cameron CCI2-L Gelding Bowman Sarah Altus Louvo Quitus Louvo Vanille du Tertre J’T’Adore Selle Francais Sarah Bowman M. -
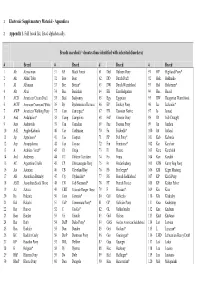
Electronic Supplementary Material - Appendices
1 Electronic Supplementary Material - Appendices 2 Appendix 1. Full breed list, listed alphabetically. Breeds searched (* denotes those identified with inherited disorders) # Breed # Breed # Breed # Breed 1 Ab Abyssinian 31 BF Black Forest 61 Dul Dülmen Pony 91 HP Highland Pony* 2 Ak Akhal Teke 32 Boe Boer 62 DD Dutch Draft 92 Hok Hokkaido 3 Al Albanian 33 Bre Breton* 63 DW Dutch Warmblood 93 Hol Holsteiner* 4 Alt Altai 34 Buc Buckskin 64 EB East Bulgarian 94 Huc Hucul 5 ACD American Cream Draft 35 Bud Budyonny 65 Egy Egyptian 95 HW Hungarian Warmblood 6 ACW American Creme and White 36 By Byelorussian Harness 66 EP Eriskay Pony 96 Ice Icelandic* 7 AWP American Walking Pony 37 Cam Camargue* 67 EN Estonian Native 97 Io Iomud 8 And Andalusian* 38 Camp Campolina 68 ExP Exmoor Pony 98 ID Irish Draught 9 Anv Andravida 39 Can Canadian 69 Fae Faeroes Pony 99 Jin Jinzhou 10 A-K Anglo-Kabarda 40 Car Carthusian 70 Fa Falabella* 100 Jut Jutland 11 Ap Appaloosa* 41 Cas Caspian 71 FP Fell Pony* 101 Kab Kabarda 12 Arp Araappaloosa 42 Cay Cayuse 72 Fin Finnhorse* 102 Kar Karabair 13 A Arabian / Arab* 43 Ch Cheju 73 Fl Fleuve 103 Kara Karabakh 14 Ard Ardennes 44 CC Chilean Corralero 74 Fo Fouta 104 Kaz Kazakh 15 AC Argentine Criollo 45 CP Chincoteague Pony 75 Fr Frederiksborg 105 KPB Kerry Bog Pony 16 Ast Asturian 46 CB Cleveland Bay 76 Fb Freiberger* 106 KM Kiger Mustang 17 AB Australian Brumby 47 Cly Clydesdale* 77 FS French Saddlebred 107 KP Kirdi Pony 18 ASH Australian Stock Horse 48 CN Cob Normand* 78 FT French Trotter 108 KF Kisber Felver 19 Az Azteca -

A Timeline of the History of the Friesian Horse
The Friesian Fall 1999 A Time-line of the History of the Friesian Horse Compiled by Laurie M. Kasperek involved in their breeding F0 II owmg· is· a lime· -1me· of many mterestmg· • histoncal. events related to the history• o ft eh Fnesian · · h° rse •the places. ' and the people important to their survival. It is by no means a complete work. More infonnation and interesting stones may be found by studying the references cited at the end of this article. The accuracy of this time-line is based on the works cited. 1500- 1600 Arabian blood introduced to the horse that descended from &mys rohystus. through Andalusian horses of Spain · 1526 Hungarian King Louis II used heavy Friesian horses in battle against the Turks. 1568 Etches by Stradanus, showing a Friesian stallion in the stables of Don Juan of Austna. 1624 Electoral Prince George William of Prussia imported Friesian horses. 1625 Friesian horses were being exported to New Amsterdam (the future New York City): . 1664 The Dutch were forced to leave New Amsterdam to the English. The purebred Fnes1an horse was quickly lost. I 700's Friesian sjees came into use . Built in the Rococo style. 1771 The stud at Kladrub imported Fricsian horses. 1795 -1796 Ads in New York City newspapers speak of trotters of'Dutch' descent. I 700-I 800's Friesians horses were popular as a trotting horse for short distances. 1823 King William I started horse races in Leeuwarden, to be held every successive year. This became known as the "King's-Golden-Whip-Day" due to the prize. -

Auction Schedule Friday, May 2, 8:00 A.M
auction schedule Friday, May 2, 8:00 a.m. Barn available for check-in 4:30 p.m. Stallion Presentation 6:30 p.m. Sale of Catalog Horses 1-46 Hitching and driving of horses Fri. afternoon and Sat. morning. Saturday, May 3, 8:00 a.m. Sale of Cataloged Horses 101-254 Location of Sale: Mt Hope Auction, 8076 SR 241, Millersburg, OH 44654 (In the town of Mt. Hope) Auctioneers: Steve Andrews and Todd Woodruff Pedigrees: Loren Beachy, Goshen, IN Ringmen: Arlen Yoder, Lynn Neuenschwander, Leroy Miller Ryan Yoder, Aden Yoder, and Dennis Mast Jr., Raymond Miller Manager: Thurman & Chester Mullet 330-674-6188 (Sale Phone) Sale Committee: Allen Raber – 330-674-2890 David Beachy – 574-825-3943 Leroy Raber – 330-698-0480 Thurman Mullet – 330-674-6188 Website: www.mthopeauction.com Terms: 3% Buyers Premium on all purchases, refunded with cash and check payments. Transfers and pedigrees for all animals will be furnished to the buyers. The seller will pay the transfer fee. All buyers should bring suitable bank reference or certified check – or see us in the of- fice before buying. Visa and MasterCard accepted. U.S funds only. Sales tax will be charged on purchase unless the exempt forms or blanket certificates are signed. Checks for Horses: If papers are in order and transfers are signed along with an ok from buyer, checks will be available on day of sale. Guarantee: Each consignor to this sale will make the guarantee he desires to give his animal or animals, and will be totally responsible for that guaran- tee. -

Breed Societies and Breeding Operations Based in Other
Date of last update: 23/08/2021 BREED SOCIETIES AND BREEDING OPERATIONS BASED IN OTHER EU MEMBER STATES WHICH HAVE OFFICIALLY NOTIFIED THEIR ACTIVITIES IN THE REPUBLIC OF IRELAND IN LINE WITH ARTICLE 12 AND ARTICLE 64(6) OF REGULATION (EU) 2016/1012 Name of Member State Species Name and Contact Name of the where the breeding Detail Breed organization is recognised Denmark Porcine Landbrug & Fødevarer Dansk Landrace (DanAvl) Danish Agriculture & Food Dansk Yorkshore Council (DanAvl) Dansk Duroc Axeltorv 3 DK 1609 København V +45 3339 4000 [email protected] https://www.lf.dk/ Denmark Hybrid Breeding Pigs Landbrug & Fødevarer DanBred Hybrid (DanAvl) Danish Agriculture (based on Dansk & Food Landrace, Dansk Council (DanAvl) Yorkshire, and Dansk Axeltorv 3 Duroc) DK 1609 København V +45 3339 4000 [email protected] https://www.lf.dk/ Germany Equine Deutsche Quarter Horse Association e.V. American Quarter https://tgrdeu.genres.de/veroeffentlichungvoninformationengema Horse esstierzuchtrecht/zuechtervereinigungen Deutsche Quarter Horse Association e.V. Daimlerstrasse 22 63741 Aschaffenburg phone:+49 (0) 6021 58459 0 Fax:+49 (0) 6021 58459 79 E-Mail: info@)dqha.de Internet: http://www.dqha.de Germany Equine Westfälisches Pferdestammbuch e.V. Westfälisches Reitpferd https://tgrdeu.genres.de/veroeffentlichungvoninformationengema esstierzuchtrecht/zuechtervereinigungen Westfälisches Pferdestammbuch e.V. Sudmühlenstraße 33 48157 Münster-Handorf phone: +49 (0) 251 32809 0 Fax: +49 (0) 251 32809 24 E-Mail: [email protected] Internet: http://www.westfalenpferde.de Germany Equine Verband der Züchter des Holsteiner Pferdes e.V. Holsteiner Warmblut https://tgrdeu.genres.de/veroeffentlichungvoninformationenge maesstierzuchtrecht/zuechtervereinigungen Verband der Züchter des Holsteiner Pferdes e.V. Steenbeker Weg 151 24106 Kiel phone: +49 (0) 431 3059960 Fax:+49 (0) 431 336142 E-Mail: [email protected] Internet: http://www.holsteiner-verband.de Germany Equine Springpferdezuchtverband Oldenburg International e.V. -

Breeding Programme for Oldenburg Horses
Breeding Programme for Oldenburg Horses Verband der Züchter des Oldenburger Pferdes e.V. Grafenhorststr. 5 · 49377 Vechta · Germany Telephone: 04441 -9355 -0 Fax: 04441 -9355 -99 [email protected] www.oldenburger-pferde.com Breeding Programme for Oldenburg Horses Breeding Programme for Oldenburg Horses 1. Information on the Studbook.....................................................................................................3 2. Geographical area ....................................................................................................................3 3. Size of the breeding population ................................................................................................3 4. Breeding goal ...........................................................................................................................3 5. Characteristic features and qualities .........................................................................................3 5.1. Exterior .....................................................................................................................................3 5.2. Movement incl. jumping ............................................................................................................4 5.3 Interior qualities .........................................................................................................................5 6. Selection characteristics ...........................................................................................................5 -

The Friesian Horse Has Been Called Upon Countless Times to Lend Its Unique Charisma to What a Role That Other- Wise Would Have Been, Just a Horse
e have all seem them. A noble black horse trots across the movie screen Wand while our focus is supposed to be on the actor, we are drawn to the dramatic steed on which he or she rides. Whether it be “The Eagle”, “The Mask of Zorro”, “Pillars of the Earth”, “Interview with a Vampire”, “Clash of the Titans” (2010) or the breed’s premier film, “Ladyhawke”, the Friesian horse has been called upon countless times to lend its unique charisma to what a role that other- wise would have been, just a horse. This is not the first time these versatile horses have answered the call of its human master and responded admirably. One of the oldest breeds of Northern Europe, the Frie- sian was initially a knight’s horse. Native to Friesland, a northern most province of what is now The Netherlands, the courageous and bold Friesian horse carried knights into bat- tle and with a similar commitment and de- votion to his battered and war weary master carried him back out. As battle tactics changed, so did the horse; eventually demonstrating the versatility that has become their hallmark, the Friesian adapted to the demands of his master and became the most dependable asset of an Agrarian society. Used on the farm during the week the Friesian did everything that to- day’s tractor now does. On Saturdays, when the workload was light, the Friesian then be- came a horse for entertainment, with athletic men and women competing with them in bareback trotting races. No day of rest for this horse; on Sunday the Friesian was hitched up and used to take the family, by carriage, to church and the inevitable after service visits. -

Development of Linear Profiling in the Holsteiner Horse - Practical Experience
3rd International Workshop on Linear Profiling in the Warmblood Horse 24 Feb 2017 Development of linear profiling in the Holsteiner horse - Practical experience - Dr. Astrid Weber EIP-Project Manager, Verband der Züchter des Holsteiner Pferdes e.V. 3rd International workshop on linear profiling in the Warmblood Horse on 24th February 2016 in Marbach / Germany Holstein linear system DEVELOPMENT • Since 2010: linear profiling of foals using an assessment sheet • 2014: Pilot study: linear profiling of mares at the studbook registration and mare performance test in Schleswig-Holstein using • 2015: Pilot study: linear profiling of young stallions at the preselection for licensing • 2015: Start of the project “Implementation of new sustainable strategies to promote equine health” within the EU-funding program “European innovation partnership” (EIP) → One part of the project is the extension of linear profiling A. Weber 1 3rd International Workshop on Linear Profiling in the Warmblood Horse 24 Feb 2017 Holstein linear system DEVELOPMENT Linear profiling of FOALS Since 2010: • linear profiling using an assessment sheet • every trait in the scheme will be judged (“trait by trait”) • only offspring of young or outcross stallions • extending the trait scheme in consultation with the judges (adding of new traits etc.; from 10 to 22 traits) Since 2016: • linear profiling of all presented foals • only striking traits will be recorded • using a tablet PC for data collection • personal documentation support (young breeders) Holstein linear system DEVELOPMENT Linear profiling of MARES Since 2014: • linear profiling of mares at the studbook registration and mare performance test in Schleswig-Holstein • using the mobile system of the Oldenburger Verband with an adapted trait scheme or using an assessment sheet • number of traits: 25 conformation traits 38 performance traits 36 performance traits for movement under rider • Since 2016: only striking traits will be recorded (time saving) A. -

Light Horses : Breeds and Management
' K>\.K>. > . .'.>.-\ j . ; .>.>.-.>>. ' UiV , >V>V >'>>>'; ) ''. , / 4 '''. 5 : , J - . ,>,',> 1 , .\ '.>^ .\ vV'.\ '>»>!> ;;••!>>>: .>. >. v-\':-\>. >*>*>. , > > > > , > > > > > > , >' > > >»» > >V> > >'» > > > > > > . »v>v - . : . 9 '< TUFTS UNIVERSITY LIBRARIES 3 9090 014 661 80 r Family Libra;-, c veterinary Medium f)HBnf"y Schoo Ve' narv Medicine^ Tu iiv 200 Wesuc . ,-<oao Nerth Graft™ MA 01538 kXsf*i : LIVE STOCK HANDBOOKS. Edited by James Sinclair, Editor of "Live Stock Journal" "Agricultural Gazette" &c. No. II. LIGHT HORSES. BREEDS AND MANAGEMENT BY W. C. A. BLEW, M.A, ; WILLIAM SCARTH DIXON ; Dr. GEORGE FLEMING, C.B., F.R.C.V.S. ; VERO SHAW, B.A. ; ETC. SIZKZTJBI ZEiZDITIOILT, le-IEJ-VISIEID. ILLUSTRATED. XonDon VINTON & COMPANY, Ltd., 8, BREAM'S BUILDINGS, CHANCERY LANE, E.C. 1919. —— l°l LIVE STOCK HANDBOOKS SERIES. THE STOCKBREEDER'S LIBRARY. Demy 8vo, 5s. net each, by post, 5s. 6d., or the set of five vols., if ordered direct from the Publisher, carriage free, 25s. net; Foreign 27s. 6d. This series covers the whole field of our British varieties of Horses, Cattle, Sheep and Pigs, and forms a thoroughly practical guide to the Breeds and Management. Each volume is complete in itself, and can be ordered separately. I. —SHEEP: Breeds and Management. New and revised 8th Edition. 48 Illustrations. By John Wrightson, M.R.A.C., F.C.S., President of the College of Agriculture, Downton. Contents. —Effects of Domestication—Long and Fine-woolled Sheep—British Long-woolled Sheep—Border Leicesters—Cotswolds—Middle-woolled—Mountain or Forest—Apparent Diff- erences in Breeds—Management—Lambing Time— Ordinary and Extraordinary Treatment of Lambs—Single and Twin Lambs—Winter Feeding—Exhibition Sheep—Future of Sheep Farm- ing—A Large Flock—Diseases. -

29. Mecklenburger Körtage
29. Mecklenburger Körtage 6$1'2.$1 *HN|UWHU0HFNOHQEXUJHUY6XUSULFH /DQGHVFKDPSLRQGHU'UHVVXUSIHUGH SODW]LHUWLP%&+)LQDOHLQ:DUHQGRUIXQG :RUOG%UHHGLQJ'UHVVDJH&KDPSLRQVKLS =-HDFFRX6XVDQQHYDQ3DV %*HVWW*DQVFKRZ 29. Mecklenburger Körtage 29. Mecklenburger ±1RYHPEHU LP/DQGJHVWW 5HGH¿Q1 IHR PLUS AN NÄHE. Rundum-Schutz für Pferd und Reiter. Partnerschaft für den Erfolg: Reiterin Sandra Lambertus und R+V-Fachberaterin Maximilia Scheubert Sprechen Sie mit uns! R+V Allgemeine Versicherung AG, BD Agrar - Herr Ralf Mahnke Lange Straße 1A 18055 Rostock www.agrarkompetenzzentrum.ruv.de Tel.: 0172-4572574 Fax: 0611-182273269 2 1 GRUSSWORT Sehr geehrte Damen und Herren! Zu den 29. Mecklenburger Körtagen begrüße ich alle Züchter und Pferdefreunde aus Mecklenburg- Vorpommern, den benachbarten Bundesländern und dem Ausland! Mecklenburger Pferde sorgen auch 2019 interna- tional für Schlagzeilen. Der 11-jährige, in Redefin von Gernot Wascher gezogene Carlchen W hat sich zum Weltklasse-Springpferd entwickelt und gehört zur erfolgreichen US-Equipe (Seite 3). Sein Vater Chacco-Blue ist zum zweiten Mal aktuell die Nr. 1 der Springpferde-Vererber im Ranking der World Breeding Association for Sporthorses (WBFSH). Er ist auch Großvater eines weiteren Großverdieners aus Mecklenburger Zucht. Cortney- Cox v. Carlo (ZG Eppinger/Puskeiler) ist mit Pius Schwizer weltweit in 5-Sterne- Springprüfungen erfolgreich (Seite 5). Im aktuellen WBFSH-Ranking nimmt der Mecklenburger Pferdezuchtverband mit seinen Springpferden Platz 16 von 51 Mitgliedsverbänden ein. Die Körveranstaltung in Redefin entwickelt sich immer mehr zu einem züchte- rischen Großereignis. Hier treffen sich attraktive Junghengste aus den besten Linien Europas. Viele der hier vorgestellten Hengste haben einige Jahre später in der Zucht und auch im Sport ihre Qualitäten bewiesen. Die in den letzten Jahren gekörten "dressurbetonten" Hengste feiern Erfolge in der schweren Klasse.