Belocaulus Spp.*
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Universidade Federal De Juiz De Fora Pós-Graduação Em Ciências Biológicas Mestrado Em Comportamento E Biologia Animal
UNIVERSIDADE FEDERAL DE JUIZ DE FORA PÓS-GRADUAÇÃO EM CIÊNCIAS BIOLÓGICAS MESTRADO EM COMPORTAMENTO E BIOLOGIA ANIMAL Camilla Aparecida de Oliveira Estratégia de história de vida e recaracterização morfológica Sarasinula linguaeformis (Semper, 1885) (Eupulmonata, Veronicellidae) Juiz de Fora 2019 Camilla Aparecida de Oliveira Estratégia de história de vida e recaracterização morfológica Sarasinula linguaeformis (Semper, 1885) (Eupulmonata, Veronicellidae) Dissertação apresentada ao Programa de Pós-Graduação em Ciências Biológicas, área de concentração: Comportamento e Biologia Animal da Universidade Federal de Juiz de Fora, como requisito parcial para obtenção do título de Mestre. Orientadora: Prof.ª. Drª. Sthefane D’ávila Juiz de Fora 2019 A todos que estiveram ao meu lado me apoiando e incentivando diante das dificuldades da carreira acadêmica, e incentivaram minha formação pessoal, profissional e dando-me suporte emocional. A vocês o meu eterno agradecimento! AGRADECIMENTOS Agradeço primeiramente a Deus por abençoar o meu caminho durante esse trabalho. A fé que tenho em Ti alimentou meu foco, minha força e minha disciplina. Depois aos meus amigos da Ciências Biológicas: Alexssandra Silva, Flávio Macanha, Isabel Macedo, Sue-helen Mondaini, Tayrine Carvalho, Kássia Malta e Yuri Carvalho meu eterno agradecimento, pois fizeram uma contribuição valiosa para a minha jornada acadêmica com seus conselhos, auxílio, palavras de apoio e risadas. Também agradeço a todos aqueles amigos que de forma direta ou indireta estiveram ajudando e torcendo por mim, em especial a Ana Claudia Mazetto, Ana Clara Files, Tamires Lima, Lígia Araújo, Raquel Seixas, Natália Corrêa e Carlota Augusta. Vocês foram fundamentais para minha formação. Agradeço à minha orientadora Sthefane D' ávila, que acompanhou meu percurso ao longo dos últimos anos e ofereceu uma orientação repleta de conhecimento, sabedoria e paciência. -

Invasive Alien Slug Could Spread Further with Climate Change
Invasive alien slug could spread further with climate change A recent study sheds light on why some alien species are more likely to become invasive than others. The research in Switzerland found that the alien Spanish slug is better able to survive under changing environmental conditions than the native 20 December 2012 Black slug, thanks to its robust ‘Jack-of-all-trades’ nature. Issue 311 Subscribe to free weekly News Alert Invasive alien species are animals and plants that are introduced accidently or deliberately into a natural environment where they are not normally found and become Source: Knop, E. & dominant in the local ecosystem. They are a potential threat to biodiversity, especially if they Reusser, N. (2012) Jack- compete with or negatively affect native species. There is concern that climate change will of-all-trades: phenotypic encourage more invasive alien species to become established and, as such, a better plasticitiy facilitates the understanding is needed of why and how some introduced species become successful invasion of an alien slug invaders. species. Proceedings of the Royal Society B Biological The study compared the ‘phenotypic plasticity’ of a native and a non-native slug species in Sciences. 279: 4668-4676. Europe. Phenotypic plasticity is the ability of an organism to alter its characteristics or traits Doi: (including behaviour) in response to changing environmental conditions. 10.1098/rspb.2012.1564. According to a framework used to understand invasive plants, invaders can benefit from phenotypic plasticity in three ways. Firstly, they are robust and can maintain fitness in Contact: varied, stressful situations, and are described as ‘Jack-of-all-trades’. -

Veronicella Spp.*
Veronicella spp.* *In April 2013, the family Veronicellidae, a target on the 2013 and 2014 AHP Prioritized Pest Lists, was broken down into six genera of concern, including Veronicella spp. Information in the datasheet may be at the family, genus, or species level. Information for specific species within the genus is included when known and relevant; other species may occur in the genus and are still reportable at the genus level. Portions of this document were taken Figure 1. Veronicella cubensis (Pfeiffer), (Image directly from the New Pest Response courtesy of David Robinson, USDA-APHIS-PPQ) Guidelines for Tropical Terrestrial Gastropods (USDA-APHIS, 2010a). Scientific Names Veronicella cubensis (Pfeiffer, 1840) Veronicella sloanii (Cuvier, 1817) Synonyms: Veronicella cubensis Onchidium cubense Pfeiffer, 1840, Onchidium cubensis, Veronicella cubensis Thomé [Thomé], 1975 Veronicella sloanei Vaginulus sloanei Férussac, [Férussac] Vaginulus laevis de Blainville, 1817 Common Name No common name, leatherleaf slugs Figure 2. Veronicella sloanei (Cuvier), (Image courtesy of David Robinson, USDA-APHIS-PPQ) Veronicella cubensis: Cuban slug Veronicella sloanii: Pancake slug Type of Pest Mollusk Taxonomic Position Class: Gastropoda, Order: Systellommatophora, Family: Veronicellidae Last update: May 2014 1 Reason for Inclusion in Manual CAPS Target: AHP Prioritized Pest List for FY 2011 – 2015* *Originally listed under the family Veronicellidae. Pest Description Veronicellidae are anatomically distinct from many other terrestrial slugs in that they have a posterior anus, eyes on contractile tentacles, and no pulmonate lung. The sensory tentacles are bilobed. This family also lacks a mantel cavity (Runham and Hunter, 1970). Although this family is fairly easy to tell apart from others, species within this family can be difficult to distinguish due to similar morphology between species and multiple color variations within a single species. -

Biological Inventory of Blaauw ECO Forest – NWD Plan 1560 (Township of Langley)
Biological Inventory of Blaauw ECO Forest – NWD Plan 1560 (Township of Langley): Including vertebrates, vascular plants, select non-vascular plants, and select invertebrates Curtis Abney Student #430748 April 21st, 2014 Thesis Advisor: Dr. D. Clements Co-Advisor: Prof. K. Steensma 1 Abstract Preserving the earth’s remaining biodiversity is a major conservation concern. Especially in areas where urban development threatens to take over prime species habitat, protecting green spaces can be an effective method for conserving local biodiversity. Establishing biological inventories of these green species can be crucial for the appropriate management of them and for the conservation of the species which use them. This particular study focused on a recently preserved forest plot, just 25 acres in size, which is now known as the Blaauw ECO Forest. The Blaauw ECO Forest was acquired by Trinity Western University in 2013 to serve as an ecological preserve where students and staff can conduct ecological research and conservation work. After ten months of research and over 100 hours of onsite observations, over 250 species of flora and fauna have been documented inhabiting or using the forest (e.g. as a breeding site). Throughout the duration of this study, several large mammals such as coyotes (Canis latrans), mule deer (Odocoileus hemionus), and black bears (Ursus americanus) have been documented using the forest which highlights the forest’s use as patch habitat. Several provincially blue- listed species have also been seen in the forest, the Pacific Sideband snail (Monadenia fidelis) and Northern Red-legged Frog (Rana aurora) in particular, and these species may testify to the forest’s rich ecology. -

Malacologia Vol. 56, No. 1-2 2013 Contents Full Papers
MALACOLOGIA VOL. 56, NO. 1-2 MALACOLOGIA 2013 http://malacologia.fmnh.org CONTENTS World’s Leading Malacological Journal FULL PAPERS Journal Impact Factor 1.592 (2012) Suzete R. Gomes, David G. Robinson, Frederick J. Zimmerman, Oscar Obregón & Nor- Founded in 1961 man B. Barr Morphological and molecular analysis of the Andean slugs Colosius confusus, n. sp., a newly recognized pest of cultivated flowers and coffee from Colombia, Ecuador and EDITOR-IN-CHIEF Peru, and Colosius pulcher (Colosi, 1921) (Gastropoda, Veronicellidae) ..... 1 GEORGE M. DAVIS Eva Hettenbergerová, Michal Horsák, Rashmi Chandran, Michal Hájek, David Zelený & Editorial Office Business & Subscription Office Jana Dvořáková Malacologia Malacologia Patterns of land snail assemblages along a fine-scale moisture gradient .... 31 P.O. Box 1222 P.O. Box 385 Nicolás Bonel, Lía C. Solari & Julio Lorda West Falmouth, Massachusetts 02574, U.S.A. Haddonfield, New Jersey 08033, U.S.A. Differences in density, shell allometry and growth between two populations of Lim- [email protected] [email protected] noperna fortunei (Mytilidae) from the Río de la Plata basin, Argentina ..... 43 Mariana L. Adami, Guido Pastorino & J. M. (Lobo) Orensanz Copy Editor Associate Editor Phenotypic differentiation of ecologically significant Brachidontes species co- EUGENE COAN JOHN B.BURCH occurring in intertidal mussel beds from the southwestern Atlantic ........ 59 Santa Barbara Museum of Natural History University of Michigan Santa Barbara, California, U.S.A. Ann Arbor, Michigan, U.S.A. Moncef Rjeibi, Soufia Ezzedine-Najai, Bachra Chemmam & Hechmi Missaoui [email protected] [email protected] Reproductive biology of Eledone cirrhosa (Cephalopoda: Octopodidae) in the northern and eastern Tunisian Sea (western and central Mediterranean) .. -

Moluscos Del Perú
Rev. Biol. Trop. 51 (Suppl. 3): 225-284, 2003 www.ucr.ac.cr www.ots.ac.cr www.ots.duke.edu Moluscos del Perú Rina Ramírez1, Carlos Paredes1, 2 y José Arenas3 1 Museo de Historia Natural, Universidad Nacional Mayor de San Marcos. Avenida Arenales 1256, Jesús María. Apartado 14-0434, Lima-14, Perú. 2 Laboratorio de Invertebrados Acuáticos, Facultad de Ciencias Biológicas, Universidad Nacional Mayor de San Marcos, Apartado 11-0058, Lima-11, Perú. 3 Laboratorio de Parasitología, Facultad de Ciencias Biológicas, Universidad Ricardo Palma. Av. Benavides 5400, Surco. P.O. Box 18-131. Lima, Perú. Abstract: Peru is an ecologically diverse country, with 84 life zones in the Holdridge system and 18 ecological regions (including two marine). 1910 molluscan species have been recorded. The highest number corresponds to the sea: 570 gastropods, 370 bivalves, 36 cephalopods, 34 polyplacoforans, 3 monoplacophorans, 3 scaphopods and 2 aplacophorans (total 1018 species). The most diverse families are Veneridae (57spp.), Muricidae (47spp.), Collumbellidae (40 spp.) and Tellinidae (37 spp.). Biogeographically, 56 % of marine species are Panamic, 11 % Peruvian and the rest occurs in both provinces; 73 marine species are endemic to Peru. Land molluscs include 763 species, 2.54 % of the global estimate and 38 % of the South American esti- mate. The most biodiverse families are Bulimulidae with 424 spp., Clausiliidae with 75 spp. and Systrophiidae with 55 spp. In contrast, only 129 freshwater species have been reported, 35 endemics (mainly hydrobiids with 14 spp. The paper includes an overview of biogeography, ecology, use, history of research efforts and conser- vation; as well as indication of areas and species that are in greater need of study. -

Slug: an Emerging Menace in Agriculture: a Review
Journal of Entomology and Zoology Studies 2020; 8(4): 01-06 E-ISSN: 2320-7078 P-ISSN: 2349-6800 www.entomoljournal.com Slug: An emerging menace in agriculture: A JEZS 2020; 8(4): 01-06 © 2020 JEZS review Received: 01-05-2020 Accepted: 03-06-2020 Partha Pratim Gyanudoy Das, Badal Bhattacharyya, Sudhansu Partha Pratim Gyanudoy Das All India Network Project on Bhagawati, Elangbam Bidyarani Devi, Nang Sena Manpoong and K Soil Arthropod Pests, Sindhura Bhairavi Department of Entomology, Assam Agricultural University, Jorhat, Assam, India Abstract Most of the terrestrial slugs are potential threat to agriculture across the globe. Their highly adaptive Badal Bhattacharyya nature helps them to survive in both temperate and tropical climates which is one of the major reasons of All India Network Project on its abundant species diversity. It is not only a severe problem in different seedlings of nursery and Soil Arthropod Pests, orchards, also a worry factor for the seeds of legumes sown in furrows. The whitish slimy mucus Department of Entomology, generated by this pest makes the flower and vegetables unfit for sale. However, despite of its euryphagic Assam Agricultural University, nature, very few works have been carried out on slug morphology, biology, ecology, taxonomy and its Jorhat, Assam, India management in India. This review article tries to integrate the information of economically important slug species of the world as well as India, their bio-ecology, nature of damage, favorable factors with Sudhansu Bhagawati special emphasis on eco-friendly management tactics of this particular gastropod pest. All India Network Project on Soil Arthropod Pests, Keywords: Slug, euryphagic, bio-ecology, management, gastropod pest Department of Entomology, Assam Agricultural University, Jorhat, Assam, India Introduction With a number of 80,000 to 135,000 members, mollusc ranks second largest invertebrate Elangbam Bidyarani Devi group in the world, out of which 1129 species of terrestrial molluscs are found in India [1, 2, 3]. -

Gastropoda: Stylommatophora)1 John L
EENY-494 Terrestrial Slugs of Florida (Gastropoda: Stylommatophora)1 John L. Capinera2 Introduction Florida has only a few terrestrial slug species that are native (indigenous), but some non-native (nonindigenous) species have successfully established here. Many interceptions of slugs are made by quarantine inspectors (Robinson 1999), including species not yet found in the United States or restricted to areas of North America other than Florida. In addition to the many potential invasive slugs originating in temperate climates such as Europe, the traditional source of invasive molluscs for the US, Florida is also quite susceptible to invasion by slugs from warmer climates. Indeed, most of the invaders that have established here are warm-weather or tropical species. Following is a discus- sion of the situation in Florida, including problems with Figure 1. Lateral view of slug showing the breathing pore (pneumostome) open. When closed, the pore can be difficult to locate. slug identification and taxonomy, as well as the behavior, Note that there are two pairs of tentacles, with the larger, upper pair ecology, and management of slugs. bearing visual organs. Credits: Lyle J. Buss, UF/IFAS Biology as nocturnal activity and dwelling mostly in sheltered Slugs are snails without a visible shell (some have an environments. Slugs also reduce water loss by opening their internal shell and a few have a greatly reduced external breathing pore (pneumostome) only periodically instead of shell). The slug life-form (with a reduced or invisible shell) having it open continuously. Slugs produce mucus (slime), has evolved a number of times in different snail families, which allows them to adhere to the substrate and provides but this shell-free body form has imparted similar behavior some protection against abrasion, but some mucus also and physiology in all species of slugs. -

Threatened Freshwater and Terrestrial Molluscs
Biodiversity Journal, 2011, 2 (2): 59-66 Threatened freshwater and terrestrial molluscs (Mollusca, Gastropoda et Bivalvia) of Santa Catarina State, Southern Brazil: check list and evaluation of regional threats A. Ignacio Agudo-Padrón Project “Avulsos Malacológicos”, Caixa Postal (P.O. Box) 010, 88010-970, Centro, Florianópolis, Santa Catarina, SC, Brasil; [email protected]; http://www.malacologia.com.br ABSTRACT A total of nineteen continental native mollusc species are confirmed for the Santa Catarina State (SC) (organized in ten Genera and seven Families), one aquatic Prosobranchia/Caenogastropoda (Ampullariidae), six Pulmonata terrestrial gastropods (one Ellobiidae, three Megalobulimidae and two micro-snails – Charopidae and Streptaxidae) and twelve freshwater mussels (eight Mycetopodidae and four Hyriidae). These species are designated by the International Union for Conservation of the Nature – IUCN as follows: seven as "Vulnerable", six "In Danger" and six “Without Category Established”. The general regional threats that these species are subjected to are briefly analyzed. KEY WORDS Biodiversity, Continental mollusc fauna, Threatened species, Santa Catarina State, Southern Brazil region Received 18.02.2011; accepted 12.04.2011; printed 30.06.2011 INTRODUCTION access is quite restricted and permitted only to researchers; this besides four “National In spite of prodigious scientific and Ecological Parks” within the jurisdiction of the technological progress in recent years, in same State. throughout Brazil and other Neotropical -

Slugs (Of Florida) (Gastropoda: Pulmonata)1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. EENY-087 Slugs (of Florida) (Gastropoda: Pulmonata)1 Lionel A. Stange and Jane E. Deisler2 Introduction washed under running water to remove excess mucus before placing in preservative. Notes on the color of Florida has a depauparate slug fauna, having the mucus secreted by the living slug would be only three native species which belong to three helpful in identification. different families. Eleven species of exotic slugs have been intercepted by USDA and DPI quarantine Biology inspectors, but only one is known to be established. Some of these, such as the gray garden slug Slugs are hermaphroditic, but often the sperm (Deroceras reticulatum Müller), spotted garden slug and ova in the gonads mature at different times (Limax maximus L.), and tawny garden slug (Limax (leading to male and female phases). Slugs flavus L.), are very destructive garden and greenhouse commonly cross fertilize and may have elaborate pests. Therefore, constant vigilance is needed to courtship dances (Karlin and Bacon 1961). They lay prevent their establishment. Some veronicellid slugs gelatinous eggs in clusters that usually average 20 to are becoming more widely distributed (Dundee 30 on the soil in concealed and moist locations. Eggs 1977). The Brazilian Veronicella ameghini are round to oval, usually colorless, and sometimes (Gambetta) has been found at several Florida have irregular rows of calcium particles which are localities (Dundee 1974). This velvety black slug absorbed by the embryo to form the internal shell should be looked for under boards and debris in (Karlin and Naegele 1958). -
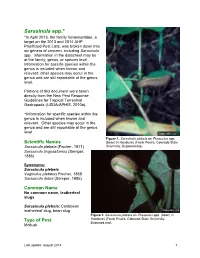
Sarasinula Spp.*
Sarasinula spp.* *In April 2013, the family Veronicellidae, a target on the 2013 and 2014 AHP Prioritized Pest Lists, was broken down into six genera of concern, including Sarasinula spp. Information in the datasheet may be at the family, genus, or species level. Information for specific species within the genus is included when known and relevant; other species may occur in the genus and are still reportable at the genus level. Portions of this document were taken directly from the New Pest Response Guidelines for Tropical Terrestrial Gastropods (USDA-APHIS, 2010a). *Information for specific species within the genus is included when known and relevant. Other species may occur in the genus and are still reportable at the genus level. Figure 1. Sarasinula plebeia on Phaseolus spp. Scientific Names (bean) in Honduras (Frank Peairs, Colorado State Sarasinula plebeia (Fischer, 1871) University, Bugwood.org). Sarasinula linguaeformis (Semper, 1885) Synonyms: Sarasinula plebeia Vaginulus plebeius Fischer, 1868 Sarasinula dubia (Semper, 1885) Common Name No common name, leatherleaf slugs Sarasinula plebeia: Caribbean leatherleaf slug, bean slug Figure 2. Sarasinula plebeia on Phaseolus spp. (bean) in Honduras (Frank Peairs, Colorado State University, Type of Pest Bugwood.org). Mollusk Last update: August 2014 1 Taxonomic Position Class: Gastropoda, Order: Systellommatophora, Family: Veronicellidae Reason for Inclusion in Manual CAPS Target: AHP Prioritized Pest List for FY 2011 – 2015* *Originally listed under the family Veronicellidae. Pest Description Veronicellidae are anatomically distinct from many other terrestrial slugs in that they have a posterior anus, eyes on contractile tentacles, and no pulmonate lung. The sensory tentacles are bilobed. This family also lacks a mantel cavity (Runham and Hunter, 1970). -

Proceedings of the Biological Society of Washington 110(4):520-536
PROCEEDINGS OF THE BIOLOGICAL SOCIETY OF WASHINGTON 110(4):520-536. 1997. Annotated list of Veronicellidae from the collections of the Academy of Natural Sciences of Philadelphia and the National Museum of Natural History, Smithsonian Institution, Washington, D.C., U.S.A. (Mollusca: Gastropoda: Soleolifera) Jose W. Thome, Patricia H. dos Santos, and Luciana Pedott Laboratories de Malacologia, Instituto de Biociencias, PUCRS, Av. Ipiranga, 6681, predio 12; 90619-900 Porto Alegre, RS-Brazil. Abstract.—The list of veronicellid slugs presented in this paper is restricted to species identified by criteria previously proposed by the first author. We were able to distinguish 30 species or subspecies, classified in 12 genera. In- cluded are the following: Belocaulus angustipes; Colosius propinquus; C. pulcher; Diplosolenodes occidentalism D. olivaceus; Heterovaginina peruviana; Laevicaulis alte (with new illustrations); L. natalensis brauni; L. stuhlmanni (with new illustrations); Latipes cnidicaulus; Leidyula dissimilis; L. floridana (with new data and illustration); L. goodfriendi; L. kraussi; L. moreleti; L. portoricensis; L. trichroma; Phyllocaulis gayi; P. soleiformis; Sarasinula du- bia; S. linguaeformis; S. plebeia; Simrothula columbiana; S. prismatica; Va- ginulus taunaisii; Veronicella bahamensis; V. cubensis; V. davisi; V. sloanei; V. tenax. The family Veronicellidae Gray, 1840 groups of characteristics to be analyzed. He comprises terrestrial mollusks without shell, comments on the characteristics tradition- commonly called slugs. The distribution is ally used and is of the opinion that there are pantropical and over 300 specific names not enough to determine conclusively the have been registered, most of them syn- species, and that they do not permit a con- onyms (Hoffmann 1925, Forcart 1953, sistent phylogenetic classification.