Origin Al Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
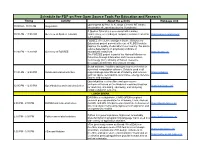
Schedule for FDP on Free Open Source Tools for Education and Research Timing Activity About the Activity Webpage Link Opening Talk by Prof
Schedule for FDP on Free Open Source Tools For Education and Research Timing Activity About the activity Webpage Link Opening talk by Prof. D. K. Singh (Director BIT Sindri), 10:00AM - 10:30 AM Inaguration Introdcution of Expert speaker by Coordinator A Spoken Tutorial is a screencast with running 10:30 AM - 11:00 AM Overview of Spoken Tutorials commentary, a recording of computer session created for https://spoken-tutorial.org/ self learning. FOSSEE (Free/Libre and Open Source Software for Education) project promotes the use of FLOSS tools to improve the quality of education in our country. We aim to reduce dependency on proprietary software in 11:00 PM - 11:30 AM Overview of FOSSEE educational institutions. https://fossee.in/ The FOSSEE project is part of the National Mission on Education through Information and Communication Technology (ICT), Ministry of Human Resource Development (MHRD), Government of India. Scilab software, free/libre and open source reference in numerical computation software. Scilab is used in all 11:30 AM - 12:00 PM Scilab and related activities major strategic scientific areas of industry and services https://scilab.in/ such as space, aeronautics, automotive, energy, defense, finance and transport. OpenModelica is a free/libre and open-source environment based on the Modelica modeling language 12:00 PM - 12:30 PM OpenModelica and related activities https://om.fossee.in/ for modeling, simulating, optimizing, and analyzing complex dynamic systems. LUNCH BREAK DWSIM is a multiplatform, CAPE-OPEN compliant chemical process simulator for Windows, Linux, Android, 2:00 PM - 2:30 PM DWSIM and related activities macOS, and iOS. -

Marko Leino Process Simulation Unit Operation Models – Review of Open
MARKO LEINO PROCESS SIMULATION UNIT OPERATION MODELS – REVIEW OF OPEN AND HSC CHEMISTRY I/O INTERFACES Master of Science Thesis Examiner: Professor Hannu Jaakkola Examiner and topic approved by the Faculty Council of the Faculty of Business and Built Environment on 6 April 2016 i ABSTRACT MARKO LEINO: Process Simulation Unit Operation Models – Review of Open and HSC Chemistry I/O Interfaces Tampere University of Technology Master of Science Thesis, 76 pages, 29 Appendix pages April 2016 Master’s Degree Programme in Information Technology Major: Software Engineering Examiner: Professor Hannu Jaakkola Keywords: CAPE-OPEN, HSC Chemistry Sim, unit operation, process simulation Chemical process modelling and simulation can be used as a design tool in the development of chemical plants, and is utilized as a means to evaluate different design options. The CAPE- OPEN interface standards were developed to allow the deployment and utilization of process modelling components in any compliant process modelling environment. This thesis examines the possibilities provided by the CAPE-OPEN interfaces and the .NET framework to develop compliant, cross-platform process modelling components, particularly unit operations. From the software engineering point of view, a unit operation is a represen- tation of physical equipment, and contains the mathematical model of its functionality. The study indicates that the differences between the CAPE-OPEN standards and Outotec HSC Chemistry Sim are negligible at the conceptual level. On the other hand, at the implementa- tion level, the differences are quite considerable. Regardless of the simulation application be- ing used, the modelling of unit operations requires interdisciplinary skills, and creating tools and methods to ease the development of such models is well justified. -

COCOP - EC Grant Agreement: 723661 Public
Ref. Ares(2019)525674 - 30/01/2019 COCOP - EC Grant Agreement: 723661 Public Project information Project title Coordinating Optimisation of Complex Industrial Processes Project acronym COCOP Project call H2020-SPIRE-2016 Grant number 723661 Project duration 1.10.2016-31.3.2020 (42 months) Document information Deliverable number D4.6 Deliverable title Modelling guideline document and demonstration development kit (update) Version 1.0 Dissemination level Public Work package WP4 Authors VTT Contributing partners TUDO, OPT Delivery date 31.1.2019 Planned delivery month M28 Keywords Modelling, tools, workflow, digital maturity, human factors This project has received European Union’s Horizon 2020 research and innovation funding under grant agreement No 723661. COCOP - EC Grant Agreement: 723661 Public This project has received European Union’s Horizon 2020 research and innovation funding under grant agreement No 723661. COCOP - EC Grant Agreement: 723661 Public VERSION HISTORY Version Description Organisation Date 0.1 Skeleton VTT 22.11.18 0.2 Added MOSAIC VTT 22.11.18 0.3 Digital Maturity, simulators, HF Milestones VTT 14.12.18 0.4 Cleansing VTT 19.12.18 0.5 Executive summary & cleansing VTT 2.1.2019 0.6 Updates per review comments VTT 21.1.2019 1.0 Final checks VTT 28.1.2019 VERSION HISTORY COCOP - EC Grant Agreement: 723661 Public EXECUTIVE SUMMARY This deliverable continues the work reported in deliverable D4.4 "Modelling guideline document and demonstration development kit". The work has progressed on two fronts, simulation tools supporting COCOP and the COCOP implementation workflow. On the former, we present a review of numerous simulators and delve deeper into the most promising ones. -

Course Structure and Curriculum for S.Y. B.TECH
Vishwakarma Institute of Technology Issue 01 : Rev No. 1 : Dt. 01/07/18 Bansilal Ramnath Agarwal Charitable Trust’s Vishwakarma Institute of Technology (An Autonomous Institute affiliated to Savitribai Phule Pune University) Structure & Syllabus of Second Year B.Tech. (Instrumentation and Control Engineering) Pattern ‘B20’ Effective from Academic Year 2020-21 Prepared by: - Board of Studies in Instrumentation & Control Engineering Approved by: - Academic Board, Vishwakarma Institute of Technology, Pune Signed by Chairman – BOS Chairman – Academic Board Structure and Syllabus of S.Y. B.Tech. Instrumentation Engineering. Pattern B-20, A.Y. 2020-21 Page No. 1 out of 31 Vishwakarma Institute of Technology, Pune Issue 01 : Rev No. 1 : Dt. 01/07/18 Contents Sr. No. Title Page No. 1 Vision, Mission of Institute and Department 3 2 PEOs and POs 4 3 PSOs 5 4 Course Structure 6 5 ‘Separator’ - Semester I 7 6 Course Syllabi for courses - Semester I (Module-IV) 8 6.1 IC2202 S1 Data Structures 8 6.2 IC2204 S2 Computer Architecture and Operating System 14 6.3 IC2206 S3 Signal and Image Processing 22 6.4 IC2208 S4 Engineering Design - II 28 6.5 IC2210 S5 Software Design - II 30 6.6 IC2212 S6 Software Development Project - II 31 6.7 IC2214 S7 Engineering Design and Innovation - IV 32 Structure and syllabus of S.Y. B.Tech. Instrumentation Engineering. Pattern B-20, A.Y. 2020-21 Page 1 of 32 Vishwakarma Institute of Technology, Pune Issue 01 : Rev No. 1 : Dt. 01/07/18 Vision statement of Institute To be globally acclaimed Institute in Technical Education and Research for holistic Socio-economic development Mission statement of Institute . -

SASTRA FOSSEE CENTRE SASTRA Thanjavur Tamil Nadu 613 401
SASTRA FOSSEE CENTRE SASTRA Thanjavur Tamil Nadu 613 401 PROCESS SIMULATION USING DWSIM: A Free and Open Source Chemical Process Simulator By Dr. P. R. Naren Senior Assistant Professor, Chemical Engineering E-mail: [email protected] School of Chemical and Biotechnology SASTRA Thanjavur 613 401 Tamil Nadu Level: Basic Version: v02 December 2017 This manual uses DWSIM ver 5.1 (Class UI) ii | Page PREFACE The manual “Process Simulation Using DWSIM” presents a set of eleven basic exercises using a free and open source chemical process simulator “DWSIM” and can be utilized to establish process simulation laboratory as part of undergraduate chemical engineering degree or in allied degree curriculum. Simulation covers topics across the broad spectrum of chemical engineering courses covering mixing, reaction, phase equilibrium, heat and mass transfer operations. The problem statements are rightly placed at the beginner’s level with each exercise complete in terms of sufficient instructions that enable the learner to perform the exercise with ease on their own. Supplementary self-learning exercises are also provided for simulation experiments to further aid a curious learner. This manual is the second version brought out by the SASTRA FOSSEE Centre, a partner with FOSSEE, IIT-B. At this juncture, I thank Prof Kannan M. Moudgalya, Professor, IIT-B for initiating me into FOSSEE tools and helping to establish the FOSSEE at SASTRA. I also owe my sincere thanks to my earlier students G. Jayaram and A. S. Ragul, who interned at FOSSEE and developed the version 1 of this manual. I believe the manual is helpful and can meet its intended objective. -
Automatised Implementation of CAPE-OPEN Interfaces: a Workfow for Equation-Based Flowsheet Modelling
Automatised Implementation of CAPE-OPEN Interfaces: A Workfow for Equation-Based Flowsheet Modelling vorgelegt von M.Sc. Gregor Stefan Tolksdorf von der Fakultät III - Prozesswissenschaften der Technischen Universität Berlin zur Erlangung des akademischen Grades Doktor der Ingenieurwissenschaften - Dr.-Ing. - genehmigte Dissertation Promotionsausschuss: Vorsitzender: Prof. Dr.-Ing. Felix Ziegler Gutachter: Prof. Dr.-Ing. habil. Jens-Uwe Repke Gutachter: Dr. Richard Baur Gutachter: Prof. Dr.-Ing. habil. Prof. h.c. Dr. h.c. Günter Wozny Tag der wissenschaftlichen Aussprache: 30.09.2019 Berlin 2019 meinen Eltern Vorwort Die vorliegende Arbeit entstand während meiner Tätigkeit als wissenschaftlicher Mitarbeiter am “Fachgebiet Dynamik und Betrieb technischer Anlagen” (DBTA) der Technischen Universität Berlin. Von Mitte 2013 bis Ende 2017 wurde meine Forschung im Rahmen der Exzellenzinitiative des Bundes und der Länder im Exzellenzcluster “Unifying Concepts in Catalysis” (UNICAT) gefördert. Ich möchte diese Stelle nutzen, um mich noch einmal bei meinen Gutachtern Prof. Wozny, Dr. Baur und Prof. Repke zu bedanken. Der Übergang der Profes- sur am Fachgebiet DBTA von Prof. Wozny, der das Fachgebiet bis 2015 über 22 Jahre lang geleitet hat, zu Prof. Repke, der die Nachfolge im Jahr 2016 übernom- men hat, machte meine Situation etwas komplizierter. Ich bedanke mich daher bei Prof. Repke für das Vertrauen, es mir fast bedingungslos zu ermöglichen, meine angefangene Arbeit erfolgreich zu beenden. Ich danke Dr. Baur für seinen Blick von außerhalb des Unibetriebs auf meine Arbeit und seine Kommentare im Zusammenhang mit meiner Forschung. Mein größter Dank gilt Prof. Wozny, der es mit seiner menschlichen, integren Art geschaft hat, mich davon zu überzeu- gen, als wissenschaftlicher Mitarbeiter eine Promotion anzustreben, in der ich mein Wissen und meine Fähigkeiten in der Schnittstelle von Informatik und Ver- fahrenstechnik praktisch anwenden kann. -

Thermodynamic and Economic Analyses of Power Generation from Gas Turbine Exhaust
Transactions of the TSME (2021) Vol. 9, No. 1 Journal of Research and Applications in Mechanical Engineering ISSN: 2229-2152 (Print); 2697-424x (Online) Paper No. JRAME-21-9-007 [DOI: 10.14456/jrame.2021.7] Research Article THERMODYNAMIC AND ECONOMIC ANALYSES OF POWER GENERATION FROM GAS TURBINE EXHAUST T. Kittijungjit1 ABSTRACT: P. Lapchit1 This paper presents the development of a thermodynamic model of natural gas T. Klamrassamee2 production processes on the offshore platform by applying Organic Rankine Y. Sukjai1,* Cycle (ORC) technology to the plant. This research considered the Y. Laoonual1 thermodynamic performance and economic feasibility of using ORC for 1Department of Mechanical Engineering, Faculty of electricity production from exhaust gas generated by gas turbine engines which Engineering, King Mongkut’s were used as prime movers to drive natural gas compressors. According to the University of Technology Thonburi, Process & Instrumentation Diagram (P&ID), the process of recovering waste 126 Pracha Uthit Rd., Bang Mod, heat from Booster Compression and Sales Gas Compression and Export modules Thung Khru, Bangkok 10140, Thailand from the entire gas production processes of the plant has been modeled in 2Mobility & Vehicle Technology DWSIM program which is an open-source chemical process simulation. The Research Center, King Mongkut’s simulation result showed that the exhaust gas had a temperature at 527 oC at University of Technology Thonburi, Booster Compression and 504 oC at Sale Gas Compression and Export. The ORC 126 Pracha Uthit Rd., Bang Mod, was designed as a simple cycle with no degree of superheating and subcooling Thung Khru, Bangkok 10140, Thailand due to limited area of the offshore platforms. -
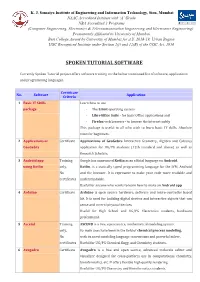
Spoken Tutorial Software
K. J. Somaiya Institute of Engineering and Information Technology, Sion, Mumbai NAAC Accredited Institute with ‘A’ Grade NBA Accredited 3 Programs (Computer Engineering, Electronics & Telecommunication Engineering and Electronics Engineering) Permanently Affiliated to University of Mumbai Best College Award by University of Mumbai for A.Y. 2018-19, Urban Region UGC Recognized Institute under Section 2(f) and 12(B) of the UGC Act, 1956 SPOKEN TUTORIAL SOFTWARE Currently Spoken Tutorial project offers software training on the below mentioned list of software, applications and programming languages. Certificate No. Software Application Criteria 1 Basic IT Skills Learn how to use package · The Linux operating system · LibreOffice Suite - for basic Office applications and · Firefox web browser - to browse the internet safely This package is useful to all who wish to learn basic IT skills. Absolute must for beginners. 2 Applications of Certificate Applications of GeoGebra Interactive Geometry, Algebra and Calculus GeoGebra application for UG/PG students (12th standard and above) as well as Research Scholars. 3 Android app Training Google has announced Kotlin as an official language on Android. using Kotlin only, Kotlin, is a statically typed programming language for the JVM, Android No and the browser. It is expressive to make your code more readable and certificates understandable. Useful for anyone who wants to learn how to make an Android app 4 Arduino Certificate Arduino is open source hardware, software and micro-controller based kit. It is used for building digital devices and interactive objects that can sense and control physical devices. Useful for High School and UG/PG Electronics students, hardware professional. -

Process-Simulation-Aliran-Gas-Alam-Pada-Pipeline-Dengan-DWSIM.Pdf
PT. PUSTEK E&T REDISCOVER ENGINEERING No. TN04-01-0420 Technical Note PT. Pustek E&T adalah publikasi hasil penelitian, review, Analisa teknis berdasarkan kaidah ilmu pengetahuan dan standard Industri tekait. Publikasi berikut bebas untuk diunduh dan digunakan secara umum tanpa tujuan komersial. Ditulis oleh : Muhammad Andry, ST1, Ahmad Hasnan, MT 1) Process Engineer PT. Pustek E&T Technical Note Sheet 2 / 16 1. PENDAHULUAN Gas alam merupakan salah satu komoditas terbesar untuk suplai energi dunia. Dalam alur suplai gas alam, berbagai fasilitas diperlukan baik pengolahan maupun transportasi untuk dapat digunakan oleh konsumen. Penggunaan gas alam secara umum yaitu untuk dijadikan sebagai bahan bakar maupun bahan baku pada fasilitas plant petrokimia. Rasio massa dan volume yang sangat rendah membuat suplai gas alam ke konsumen perlu dilakukan dengan berbagai cara, baik suplai gas langsung melalui pipeline atau suplai dengan transportasi dimana gas perlu pre-treatment untuk menghasilkan gas alam terkompresi (CNG) atau gas alam tercairkan (LNG). Kebutuhan akan gas alam yang meningkat baik di industri maupun rumah tangga mendorong berbagai aktivitas pembangunan fasilitas metering gas dan pipeline. Di tahap awal pembangunan pipeline, analisis aliran gas perlu dilakukan untuk memastikan gas terdistribusi dengan laju alir volume dan tekanan sesuai dengan persyaratan dari konsumen. Selain itu, velocity aliran gas juga perlu dipastikan agar tidak melebihi batas maksimum sizing pipa pipeline. Analisis aliran gas dapat dilakukan dengan simulasi proses menggunakan software DWSIM. Gambar 1: Tampilan interface pada DWSIM. [email protected] | http://pustek.com Technical Note Sheet 3 / 16 2. DWSIM SOFTWARE DWSIM adalah modelling software atau lebih dikenal sebagai Computer Aided Process Engineering Software dengan kemampuan multiplatform untuk mensimulasikan proses kimia yang dapat digunakan untuk operating sistem Windows, Linux, Android, MacOS dan IOS yang bersifat Open Source, sehingga dapat digunakan baik untuk individu maupun corporate secara gratis1. -

Digital Initiatives in Higher Education by MHRD
DIGITAL INITIATIVES IN HIGHER EDUCATION Ministry of Human Resource Development Department of Higher Education Government of India Foreword The quality and access to higher education has been a subject of intense concern to all of us. The advancements in digital technologies are transforming the way educational resources are being created, disseminated and accessed across the world. Use of digital technologies in all aspects of higher education has also received a major push in India. This in turn helps the quality improvement & accessibility of Higher Education to large part of students as well as up-skilling of the teachers across the country. Digital technologies have also greatly impacted the management and governance of the institutions as well as the accreditation and ranking of the Higher Education Institutions (HEIs). Department of Higher Education, Ministry of Human Resource Development, has undertaken many initiatives for exploiting the digital technologies in improving the quality & accessibility in Higher Education as well as better tools for accreditation and grading of educational institutions. )LQDOO\ PDQ\ QHZ IURQWOLQH WHFKQRORJLHV OLNH $UWLÀFLDO ,QWHOOLJHQFH 0DFKLQH /HDUQLQJ Autonomous Systems, Cognitive Systems, etc are emerging and these are going to give many new opportunities for all of us to further improve the quality & accessibility in higher education. I sincerely believe that this Handbook on “ Digital Initiatives in Higher Education” will help \RXLQIXUWKHUGLVVHPLQDWLQJDQGJHWWLQJWKHEHQHÀWVIURPWKHVHLQLWLDWLYHVWRLPSURYH the learning outcomes of not only the students but also the lifelong learners. R. Subrahmanyam, IAS Secretary DIGITAL INITIATIVES IN HIGHER EDUCATION Ministry of Human Resource Development Department of Higher Education Government of India Preface The use of Information and Communication technology (ICT) has great potential in improving the quality of education imparted and widening the access of education throughout the country. -

DWSIM Installation Manual
DWSIM Installation Manual DWSIM Team FOSSEE, IIT Bombay August 10, 2020 System Requirements OS: Windows: 32/64-bit Vista/7/8/10 Linux: 64-bit x86 Ubuntu 12.04 or newer / 32-bit armhf Raspbian/Ubuntu MATE macOS: 10.7 (OS X Lion) or newer Software: Microsoft .NET Framework 4.6.1 or newer (Windows) Mono Framework 6.8.0 or newer (Linux) CPU: 1.0 GHz dual-core processor (minimum) Memory: 2 GB RAM Graphics Card: 128 MB with OpenGL hardware acceleration Display: 1280x800 display resolution is recommended as a minimum Disk space: 500 MB for program files 1 1 The procedure to install DWSIM on Windows OS 1. Please make sure that you meet the system requirements to be able to download and install DWSIM. 2. Open your default web browser. 3. In the address bar, type the following URL and press Enter. https://sourceforge.net/projects/dwsim/files/DWSIM/ 4. Click on the folder with latest version. Currently it is DWSIM 6.1. 5. Click on the folder with latest update. Currently it is Update 1. 6. Click on "DWSIM bin v61u1 setup win7win8win10 64bit.exe" (if it is 64 bit Operating System) Click on "DWSIM bin v61u1 setup win7win8win10 32bit.exe" (if it is 32 bit Operating System) 7. The .exe file gets downloaded and saved in the Downloads folder. 8. Go to Downloads folder and double click on .exe file. 9. In User Account Control window, click Yes. 10. In Installer Language window,Click OK. 11. DWSIM 6.1 Update 1 setup window appears. Click Next.