Reproductive Behaviour of Neurobasis Kaupi (Odonata: Calopterygidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mechanism of the Wing Colouration in the Dragonfly Zenithoptera Lanei
Journal of Insect Physiology 81 (2015) 129–136 Contents lists available at ScienceDirect Journal of Insect Physiology journal homepage: www.elsevier.com/locate/jinsphys Mechanism of the wing colouration in the dragonfly Zenithoptera lanei (Odonata: Libellulidae) and its role in intraspecific communication ⇑ Rhainer Guillermo-Ferreira a,b,c, , Pitágoras C. Bispo b, Esther Appel c, Alexander Kovalev c, Stanislav N. Gorb c a Department of Hydrobiology, Federal University of São Carlos, Rod. Washington Luis, km 235, São Carlos, São Paulo, Brazil b Department of Biological Sciences, São Paulo State University, Av. Dom Antônio 2100, Assis, São Paulo, Brazil c Department of Functional Morphology and Biomechanics, Zoological Institute, Kiel University, Am Botanischen Garten 1-9, D-24098 Kiel, Germany article info abstract Article history: Zenithoptera dragonflies are known for their remarkable bluish colouration on their wings and unique Received 29 July 2014 male behaviour of folding and unfolding their wings while perching. However, nothing is known about Received in revised form 14 July 2015 the optical properties of such colouration and its structural and functional background. In this paper, Accepted 15 July 2015 we aimed to study the relationship between the wing membrane ultrastructure, surface microstructure Available online 17 July 2015 and colour spectra of male wings in Zenithoptera lanei and test the hypothesis that colouration functions as a signal in territorial fights between males. The results show that the specific wing colouration derives Keywords: from interference in alternating layers of melanized and unmelanized cuticle in the wing membrane, Colour combined with diffuse scattering in two different layers of wax crystals on the dorsal wing surface, Optics Iridescence one lower layer of long filaments, and one upper layer of leaf-shaped crystals. -

The Superfamily Calopterygoidea in South China: Taxonomy and Distribution. Progress Report for 2009 Surveys Zhang Haomiao* *PH D
International Dragonfly Fund - Report 26 (2010): 1-36 1 The Superfamily Calopterygoidea in South China: taxonomy and distribution. Progress Report for 2009 surveys Zhang Haomiao* *PH D student at the Department of Entomology, College of Natural Resources and Environment, South China Agricultural University, Guangzhou 510642, China. Email: [email protected] Introduction Three families in the superfamily Calopterygoidea occur in China, viz. the Calo- pterygidae, Chlorocyphidae and Euphaeidae. They include numerous species that are distributed widely across South China, mainly in streams and upland running waters at moderate altitudes. To date, our knowledge of Chinese spe- cies has remained inadequate: the taxonomy of some genera is unresolved and no attempt has been made to map the distribution of the various species and genera. This project is therefore aimed at providing taxonomic (including on larval morphology), biological, and distributional information on the super- family in South China. In 2009, two series of surveys were conducted to Southwest China-Guizhou and Yunnan Provinces. The two provinces are characterized by karst limestone arranged in steep hills and intermontane basins. The climate is warm and the weather is frequently cloudy and rainy all year. This area is usually regarded as one of biodiversity “hotspot” in China (Xu & Wilkes, 2004). Many interesting species are recorded, the checklist and photos of these sur- veys are reported here. And the progress of the research on the superfamily Calopterygoidea is appended. Methods Odonata were recorded by the specimens collected and identified from pho- tographs. The working team includes only four people, the surveys to South- west China were completed by the author and the photographer, Mr. -

Predictive Modelling of Spatial Biodiversity Data to Support Ecological Network Mapping: a Case Study in the Fens
Predictive modelling of spatial biodiversity data to support ecological network mapping: a case study in the Fens Christopher J Panter, Paul M Dolman, Hannah L Mossman Final Report: July 2013 Supported and steered by the Fens for the Future partnership and the Environment Agency www.fensforthefuture.org.uk Published by: School of Environmental Sciences, University of East Anglia, Norwich, NR4 7TJ, UK Suggested citation: Panter C.J., Dolman P.M., Mossman, H.L (2013) Predictive modelling of spatial biodiversity data to support ecological network mapping: a case study in the Fens. University of East Anglia, Norwich. ISBN: 978-0-9567812-3-9 © Copyright rests with the authors. Acknowledgements This project was supported and steered by the Fens for the Future partnership. Funding was provided by the Environment Agency (Dominic Coath). We thank all of the species recorders and natural historians, without whom this work would not be possible. Cover picture: Extract of a map showing the predicted distribution of biodiversity. Contents Executive summary .................................................................................................................... 4 Introduction ............................................................................................................................... 5 Methodology .......................................................................................................................... 6 Biological data ................................................................................................................... -

Zoo Og Cal S Ryey of I Dia
M l CELLANEOUS P BLI ATIO~ OCCA (0 AL PAPER O. 20 I ecords of the Zoo og cal S ryey of I dia FIELD ECOLOGY, ZOOGEOGRAPHY AND TAXONOMY OF THE ODONATA OF WESTERN HIMALAYA, INDIA By ARUN KUMAR AND MAHA8IR PRASAD Issued by the Director Zoo1ogical Survey of India, Calcutta RECORDS OFTHE Zoological Survey of India MISCELLANEOUS PUBLICATION OCCASIONAL PAPER NO. 20 FIELD ECOLOGY, ZOOGEOGRAPHY AND TAXONOMY OF THE ODONATA OF WESTERN HIMALAYA, INDIA By AruD Kumar and Mababir Prasad Northern Regional Station, Zoological Survey of Inelia, Dellra D,u, Edited by the Director, Zoological Survey of India, ('a/cult" 1981 © Copyright 1981. Government of India Published in March, 1981 PRICE: Inland: Rs. 40.00 Foreign: £ 4.50 $ 12.00 Printed in Indi~ at SAAKHHAR MUDRAN 4 Deshapran Shasmal Road Calcutta 700 033 and Published by the Controller of Publications. Civil Lines, Delhi 110006 RECORDS OFTHE Zoological Survey of India MISCELLANEOUS PUBLICATION Occasional Paper No. 20 1981 Pages 1-118 CONTENTS Page No. INTRODUCTION 1 GEOGRAPHICAL FEATURES, DIVISIONS AND CLIMATE OF WESTERN HIMALAYA 4 BRIEF DESCRIPTION OF TYPICAL OOONATA BIOTOPES IN WESTERN HIMALAYA 5 PHENOLOGY 8 KEY TO THE ODONATA OF WESTERN HIMALAYA 9 CHECK-LIST OF OOONATA OF WESTERN HIMALAYA WITH NOTES ON FIELD ECOLOGY 32 ZOOGEOGRAPHY OF ODONATA OF WESTERN HIMALAYA 67 SUMMARY 72 REFERENCES 98 FIELD ECOLOGV, ZOOGEOGRAPHY AND TAXONOMY OF THE ODONATA OF WESTERN HIMALAYA, INDIA By ARUN KUMAR AND MAHABIR PRASAD": Northern Regional Station, Zoological Survey of India, Dehra Dun (With 13 Text figures, 1 Plate and 3 Tables) INTRODUCTION Within the Indian sub-region, the Odonata Fauna of Himalaya has so far been studied most extensively. -

Identification Guide to the Australian Odonata Australian the to Guide Identification
Identification Guide to theAustralian Odonata www.environment.nsw.gov.au Identification Guide to the Australian Odonata Department of Environment, Climate Change and Water NSW Identification Guide to the Australian Odonata Department of Environment, Climate Change and Water NSW National Library of Australia Cataloguing-in-Publication data Theischinger, G. (Gunther), 1940– Identification Guide to the Australian Odonata 1. Odonata – Australia. 2. Odonata – Australia – Identification. I. Endersby I. (Ian), 1941- . II. Department of Environment and Climate Change NSW © 2009 Department of Environment, Climate Change and Water NSW Front cover: Petalura gigantea, male (photo R. Tuft) Prepared by: Gunther Theischinger, Waters and Catchments Science, Department of Environment, Climate Change and Water NSW and Ian Endersby, 56 Looker Road, Montmorency, Victoria 3094 Published by: Department of Environment, Climate Change and Water NSW 59–61 Goulburn Street Sydney PO Box A290 Sydney South 1232 Phone: (02) 9995 5000 (switchboard) Phone: 131555 (information & publication requests) Fax: (02) 9995 5999 Email: [email protected] Website: www.environment.nsw.gov.au The Department of Environment, Climate Change and Water NSW is pleased to allow this material to be reproduced in whole or in part, provided the meaning is unchanged and its source, publisher and authorship are acknowledged. ISBN 978 1 74232 475 3 DECCW 2009/730 December 2009 Printed using environmentally sustainable paper. Contents About this guide iv 1 Introduction 1 2 Systematics -

Issue 20 (2017)
IDF IDF Faunistic Studies in South-east Asian and Pacific Island Odonata Journal of the International Dragonfly Fund 1-34 Seehausen, Malte Survey of Odonata from Timor Island, with description of the female of Anax georgius (Odonata: Aeshnidae) published 10.06.2017 No. 20 ISSN 2195-4534 The International Dragonfly Fund (IDF) is a scientific society founded in 1996 for the impro- vement of odonatological knowledge and the protection of species. Internet: http://www.dragonflyfund.org/ This series intends to contribute to the knowledge of the regional Odonata fauna of the Southeas-tern Asian and Pacific regions to facilitate cost-efficient and rapid dissemination of faunistic data. Southeast Asia or Southeastern Asia is a subregion of Asia, consisting of the countries that are geo-graphically south of China, east of India, west of New Guinea and north of Austra- lia. Southeast Asia consists of two geographic regions: Mainland Southeast Asia (Indo- china) and Maritime Southeast Asia. Pacific Islands comprise of Micronesian, Melanesian and Polynesian Islands. Editorial Work: Martin Schorr, Milen Marinov and Rory Dow Layout: Martin Schorr IDF-home page: Holger Hunger Printing: Colour Connection GmbH, Frankfurt Impressum: Publisher: International Dragonfly Fund e.V., Schulstr. 7B, 54314 Zerf, Germany. E-mail: [email protected] Responsible editor: Martin Schorr Cover picture: Xiphiagrion cyanomelas Photographer: Malte Seehausen Published 10.06.2017 Survey of Odonata from Timor Island, with description of the female of Anax georgius (Odonata: Aeshnidae) Malte Seehausen Museum Wiesbaden, Naturhistorische Sammlungen, Friedrich-Ebert-Allee 2, 65185 Wiesbaden, Germany Email: [email protected] Abstract The survey is based on specimens held at Museums in Australia, Belgium and Ger- many. -

Ohio Damselfly Species Checklist
Ohio Damselfly Species Checklist Ohio has ~51 species of damselflies (Zygoptera). This is a statewide species checklist to encourage observations of damselflies for the Ohio Dragonfly Survey. Please submit photo observations to iNaturalist.org. More information can be found on our survey website at u.osu.edu/ohioodonatasurvey/ Broad Winged Damselflies (Calopterygidae) 1 Appalachian Jewelwing Calopteryx angustipennis 2 River Jewelwing Calopteryx aequabilis State Endangered 3 Ebony Jewelwing Calopteryx maculata 4 American Rubyspot Hetaerina americana 5 Smoky Rubyspot Hetaerina titia Pond Damselflies (Coenagrionidae) 6 Eastern Red Damsel Amphiagrion saucium 7 Blue-fronted Dancer Argia apicalis 8 Seepage Dancer Argia bipunctulata State Endangered 9 Powdered Dancer Argia moesta 10 Blue-ringed Dancer Argia sedula 11 Blue-tipped Dancer Argia tibialis 12 Dusky Dancer Argia translata 13 Violet Dancer Argia fumipennis violacea 14 Aurora Damsel Chromagrion conditum 15 Taiga Bluet Coenagrion resolutum 16 Turquoise Bluet Enallagma divagans 17 Hagen's Bluet Enallagma hageni 18 Boreal Bluet Enallagma boreale State Threatened 19 Northern Bluet Enallagma annexum State Threatened 20 Skimming Bluet Enallagma geminatum 21 Orange Bluet Enallagma signatum 22 Vesper Bluet Enallagma vesperum 23 Marsh Bluet Enallagma ebrium State Threatened 24 Stream Bluet Enallagma exsulans 25 Rainbow Bluet Enallagma antennatum 26 Tule Bluet Enallagma carunculatum 27 Atlantic Bluet Enallagma doubledayi 1 28 Familiar Bluet Enallagma civile 29 Double-striped Bluet Enallagma basidens -

Reproductive Behaviour and the System of Signalling in Neurobasis Chinensis (Odonata, Calopterygidae) – a Kinematic Analysis
International Journal of Odonatology, 2014 Vol. 17, No. 1, 31–52, http://dx.doi.org/10.1080/13887890.2014.881305 Reproductive behaviour and the system of signalling in Neurobasis chinensis (Odonata, Calopterygidae) – a kinematic analysis André Günthera∗, Dagmar Hilfert-Rüppellb and Georg Rüppellc aTU Bergakademie Freiberg, Institut für Biowissenschaften, Leipziger Str. 29, D-09599 Freiberg, Germany; bInstitut für Fachdidaktik der Naturwissenschaften, Technische Universität, Braunschweig, Germany; cAn der Wasserfurche 32, D-38162 Cremlingen, Germany (Received 10 December 2013; accepted 3 January 2014) The reproductive behaviour of the damselfly Neurobasis chinensis (Calopterygidae) was filmed at 300 and 600 frames per second in Thailand in spring 2009. This was subsequently viewed in slow motion for detailed analysis. Altogether we observed 26 matings at two different sites. Besides visual observations of behaviour of male–female encounters at the reproductive sites, we analysed their flight cinematographically by measuring velocity, wing beat frequency, phase relationships of fore- and hind wings, and described the flight paths of different flight manoeuvres. Wing clapping by the perched insects was analysed in detail. Also filmed were alternative reproductive behaviour and avoidance behaviour when attacked by a hunting spider. By analysing the video footage in slow motion, details of male flight with hind wings held motionless, a typical flight-style for this genus, were revealed. The significance of this behaviour in interactions with conspecifics is discussed. Keywords: reproductive behaviour; Calopterygidae; Neurobasis chinensis; Thailand; flight kinematics; high-speed videography Introduction The behaviour of the genus Neurobasis has thus far been incompletely studied. An overview of existing knowledge of all Neurobasis species by Orr and Hämäläinen (2007) is significant for the gaps it reveals in our knowledge. -

From the Ebony Jewelwing Damsel
Comp. Parasitol. 71(2), 2004, pp. 141–153 Calyxocephalus karyopera g. nov., sp. nov. (Eugregarinorida: Actinocephalidae: Actinocephalinae) from the Ebony Jewelwing Damselfly Calopteryx maculata (Zygoptera: Calopterygidae) in Southeast Nebraska, U.S.A.: Implications for Mechanical Prey–Vector Stabilization of Exogenous Gregarine Development RICHARD E. CLOPTON Department of Natural Science, Peru State College, Peru, Nebraska 68421, U.S.A. (e-mail: [email protected]) ABSTRACT: Calyxocephalus karyopera g. nov., sp. nov. (Apicomplexa: Eugregarinorida: Actinocephalidae: Actino- cephalinae) is described from the Ebony Jewelwing Damselfly Calopteryx maculata (Odonata: Zygoptera: Calopteryigidae) collected along Turkey Creek in Johnson County, Nebraska, U.S.A. Calyxocephalus gen. n. is distinguished by the form of the epimerite complex: a terminal thick disk or linearly crateriform sucker with a distal apopetalus calyx of petaloid lobes and a short intercalating diamerite (less than half of the total holdfast length). The epimerite complex is conspicuous until association and syzygy. Association occurs immediately before syzygy and is cephalolateral and biassociative. Gametocysts are spherical with a conspicuous hyaline coat. Lacking conspicuous sporoducts they dehisce by simple rupture. Oocysts are axially symmetric, hexagonal dipyramidic in shape with slight polar truncations, bearing 6 equatorial spines, 1 at each equatorial vertex and 6 terminal spines obliquely inserted at each pole, 1 at each vertex created by polar truncation. The ecology of the C. karyopera–C. maculata host–parasite system provides a mechanism for mechanical prey–vector stabilization of exogenous gregarine development and isolation. KEY WORDS: Odonata, Zygoptera, Calopteryx maculata, damselfly, Apicomplexa, Eugregarinida, Actinocephalidae, Actinocephalinae, Calyxocephalus karyopera, new genus, new species, parasite evolution, biodiversity, species isolation, vector, transmission. -

The Classification and Diversity of Dragonflies and Damselflies (Odonata)*
Zootaxa 3703 (1): 036–045 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Correspondence ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3703.1.9 http://zoobank.org/urn:lsid:zoobank.org:pub:9F5D2E03-6ABE-4425-9713-99888C0C8690 The classification and diversity of dragonflies and damselflies (Odonata)* KLAAS-DOUWE B. DIJKSTRA1, GÜNTER BECHLY2, SETH M. BYBEE3, RORY A. DOW1, HENRI J. DUMONT4, GÜNTHER FLECK5, ROSSER W. GARRISON6, MATTI HÄMÄLÄINEN1, VINCENT J. KALKMAN1, HARUKI KARUBE7, MICHAEL L. MAY8, ALBERT G. ORR9, DENNIS R. PAULSON10, ANDREW C. REHN11, GÜNTHER THEISCHINGER12, JOHN W.H. TRUEMAN13, JAN VAN TOL1, NATALIA VON ELLENRIEDER6 & JESSICA WARE14 1Naturalis Biodiversity Centre, PO Box 9517, NL-2300 RA Leiden, The Netherlands. E-mail: [email protected]; [email protected]; [email protected]; [email protected]; [email protected] 2Staatliches Museum für Naturkunde Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany. E-mail: [email protected] 3Department of Biology, Brigham Young University, 401 WIDB, Provo, UT. 84602 USA. E-mail: [email protected] 4Department of Biology, Ghent University, Ledeganckstraat 35, B-9000 Ghent, Belgium. E-mail: [email protected] 5France. E-mail: [email protected] 6Plant Pest Diagnostics Branch, California Department of Food & Agriculture, 3294 Meadowview Road, Sacramento, CA 95832- 1448, USA. E-mail: [email protected]; [email protected] 7Kanagawa Prefectural Museum of Natural History, 499 Iryuda, Odawara, Kanagawa, 250-0031 Japan. E-mail: [email protected] 8Department of Entomology, Rutgers University, Blake Hall, 93 Lipman Drive, New Brunswick, New Jersey 08901, USA. -
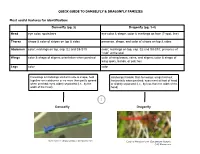
Dragonfly (Pg. 3-4) Head Eye Color
QUICK GUIDE TO DAMSELFLY & DRAGONFLY FAMILIES Most useful features for identification: Damselfly (pg. 2) Dragonfly (pg. 3-4) Head eye color; spots/bars eye color & shape; color & markings on face (T-spot, line) Thorax shape & color of stripes on top & sides presence, shape, and color of stripes on top & sides Abdomen color; markings on top, esp. S2 and S8-S10 color; markings on top, esp. S2 and S8-S10; presence of “club” at the end Wings color & shape of stigma; orientation when perched color of wing bases, veins, and stigma; color & shape of wing spots, bands, or patches Legs color color Forewings & hindwings similar in size & shape, held Hindwings broader than forewings; wings held out together over abdomen or no more than partly spread horizontally when perched; eyes meet at front of head when perched; eyes widely separated (i.e., by the or slightly separated (i.e., by less than the width of the width of the head) head) 1 Damselfly Dragonfly Vivid Dancer (Argia vivida); CAS Mazzacano Cardinal Meadowhawk (Sympetrum illotum); CAS Mazzacano DAMSELFLIES Wings narrow, stalked at base 2 Wings broad, colored, not stalked at base 3 Wings held askew Wings held together Broad-winged Damselfly when perched when perched (Calopterygidae); streams Spreadwing Pond Damsels (Coenagrionidae); (Lestidae); ponds ponds, streams Dancer (Argia); Wings held above abdomen; vivid colors streams 4 Wings held along Bluet (Enallagma); River Jewelwing (Calopteryx aequabilis); abdomen; mostly blue ponds CAS Mazzacano Dark abdomen with blue Forktail (Ischnura); tip; -

Checklist of the Dragonflies and Damselflies (Insecta: Odonata) of Bangladesh, Bhutan, India, Nepal, Pakistan and Sri Lanka
Zootaxa 4849 (1): 001–084 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Monograph ZOOTAXA Copyright © 2020 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4849.1.1 http://zoobank.org/urn:lsid:zoobank.org:pub:FFD13DF6-A501-4161-B03A-2CD143B32AC6 ZOOTAXA 4849 Checklist of the dragonflies and damselflies (Insecta: Odonata) of Bangladesh, Bhutan, India, Nepal, Pakistan and Sri Lanka V.J. KALKMAN1*, R. BABU2,3, M. BEDJANIČ4, K. CONNIFF5, T. GYELTSHEN6, M.K. KHAN7, K.A. SUBRAMANIAN2,8, A. ZIA9 & A.G. ORR10 1Naturalis Biodiversity Center, P.O. Box 9517, 2300 RA Leiden, The Netherlands. [email protected]; https://orcid.org/0000-0002-1484-7865 2Zoological Survey of India, Southern Regional Centre, Santhome High Road, Chennai-600 028, Tamil Nadu, India. 3 [email protected]; https://orcid.org/0000-0001-9147-4540 4National Institute of Biology, Večna pot 111, SI-1000, Ljubljana, Slovenia. [email protected]; https://orcid.org/0000-0002-1926-0086 5ICIMOD, GPO Box 3226 Kumalthar, Kathmandu, Nepal. [email protected]; https://orcid.org/0000-0002-8465-7127 6Ugyen Wangchuk Institute for Conservation of Environment and Research, Bumthang, Bhutan. [email protected]; https://orcid.org/0000-0002-5906-2922 7Department of Biochemistry and Molecular Biology, School of Life Sciences, Shahjalal University of Science and Technology, Sylhet 3114, Bangladesh. [email protected]; https://orcid.org/0000-0003-1795-1315 8 [email protected]; https://orcid.org/0000-0003-0872-9771 9National Insect Museum, National Agriculture Research Centre, Islamabad, Pakistan. [email protected]; https://orcid.org/0000-0001-6907-3070 10Environmental Futures Research Institute, Griffith University, Nathan, Australia.