An Overview of Interactions Between Micronutrients and of Micronutrients with Drugs, Genes and Immune Mechanisms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: the Effect on Keratinocyte Subpopulations
Acta Derm Venereol 2004; 84: 195–200 INVESTIGATIVE REPORT A Left/Right Comparison of Twice-Daily Calcipotriol Ointment and Calcitriol Ointment in Patients with Psoriasis: The Effect on Keratinocyte Subpopulations Mannon E.J. FRANSSEN, Gys J. DE JONGH, Piet E.J. VAN ERP and Peter C.M. VAN DE KERKHOF Department of Dermatology, University Medical Centre Nijmegen, The Netherlands Vitamin D3 analogues are a first-line treatment of Calcipotriol (Daivonex1,50mg/g ointment, Leo chronic plaque psoriasis, but so far, comparative clinical Pharmaceutical Products, Denmark) has been investi- studies on calcipotriol and calcitriol ointment are sparse, gated intensively during the last decade, and has proven and in particular no comparative studies are available on to be a valuable tool in the management of chronic cell biological effects of these compounds in vivo. Using plaque psoriasis. A review by Ashcroft et al. (1), based on flow cytometric assessment, we investigated whether these a large number of randomized controlled trials, showed compounds had different effects on the composition and that calcipotriol was at least as effective as potent DNA synthesis of epidermal cell populations responsible topical corticosteroids, 1a,-25-dihydroxycholecalciferol for the psoriatic phenotype. For 8 weeks, 20 patients with (calcitriol), short-contact dithranol, tacalcitol and coal psoriasis vulgaris were treated twice daily with calcipo- tar. Recently, Scott et al. (2) presented an overview of triol and calcitriol ointment in a left/right comparative studies on the use of calcipotriol ointment in the study. Before and after treatment, clinical assessment of management of psoriasis. They reconfirmed the super- target lesions was performed, together with flow cyto- ior efficacy of a twice-daily calcipotriol ointment metric analysis of epidermal subpopulations with respect regimen to the treatments as mentioned above, and to keratin (K) 10, K6, vimentin and DNA distribution. -

Table S4. List of Enzymes Directly Involved in the Anti-Oxidant Defense Response
Table S4. List of Enzymes directly involved in the anti-oxidant defense response. Gene Name Gene Symbol Classification/Pathway 6-phosphogluconate dehydrogenase 6PGD NADPH regeneration/Pentose Phosphate Glucose-6-phosphate dehydrogenase G6PD NADPH regeneration/Pentose Phosphate Isocitrate Dehydrogenase 1 IDH1 NADPH regeneration/Krebs Isocitrate Dehydrogenase 2 IDH2 NADPH regeneration/Krebs Malic Enzyme 1 ME1 NADPH regeneration/Krebs Methylenetetrahydrofolate dehydrogenase 1 MTHFD1 NADPH regeneration/Folate Methylenetetrahydrofolate dehydrogenase 2 MTHFD2 NADPH regeneration/Folate Nicotinamide Nucleotide Transhydrogenase NNT NADPH regeneration/NAD Catalase CAT Antioxidants/Catalses/free radical detoxification Glutamate-cysteine ligase catalytic subunit GCLC Antioxidants/Glutathione synthesis Glutamate-cysteine ligase modifier subunit GCLM Antioxidants/Glutathione synthesis Glutathione peroxidase1 GPx1 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione peroxidase2 GPx2 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione peroxidase3 GPx3 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione peroxidase4 GPx4 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione peroxidase5 GPx5 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione peroxidase6 GPx6 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione peroxidase7 GPx7 Antioxidants/Glutathione Peroxidases/free radical detoxification Glutathione S-transferase -

Profile of Clinical Efficacy and Safety of Topical Tacalcitol
leone 9-06-2005 14:07 Pagina 13 ACTA BIO MED 2005; 76; 13-19 © Mattioli 1885 U PDATE Profile of clinical efficacy and safety of topical tacalcitol Giovanni Leone, Alessia Pacifico Phototherapy Service, San Gallicano Dermatological Institute, IRCCS, Rome, Italy Abstract. Several topical treatments such as ointments, keratolytics, dithranol, tar, corticosteroids and Vita- min D3 analogues are commonly used in the treatment of mild and/or moderate psoriasis. These treatments can be associated with a variety of local and systemic side effects, as well as to very often unsatisfactory re- sults. The purpose of this critical review of the literature is to evaluate the efficacy and tolerability of the syn- thesis of new analogues of the Vitamin D3 Tacalcitol, which is formulated in ointment form at a concentra- tion of 4 μg/g, for the treatment of mild and/or moderate psoriasis (involvement of <20% of the surface of the skin) and to evaluate whether this drug can be used in the treatment of other skin conditions. Based on existing data in the literature, Tacalcitol is an effective drug for the topical treatment of psoriasis and is also able to ensure that the effects last over time, even after treatment has stopped. Tacalcitol is also well tolerat- ed because the onset of side effects, such as local irritation, pruriginous or burning sensations, were reported in only a small percentage of the subjects who were treated. Lastly, the marked regulatory effects it has on the proliferation and differentiation of keratinocytes, as well as on the immunocompetent cells, has led to suggestions that Tacalcitol may be used in other keratinisation disorders and in some hyperproliferative skin diseases. -

Nutrition 102 – Class 3
Nutrition 102 – Class 3 Angel Woolever, RD, CD 1 Nutrition 102 “Introduction to Human Nutrition” second edition Edited by Michael J. Gibney, Susan A. Lanham-New, Aedin Cassidy, and Hester H. Vorster May be purchased online but is not required for the class. 2 Technical Difficulties Contact: Erin Deichman 574.753.1706 [email protected] 3 Questions You may raise your hand and type your question. All questions will be answered at the end of the webinar to save time. 4 Review from Last Week Vitamins E, K, and C What it is Source Function Requirement Absorption Deficiency Toxicity Non-essential compounds Bioflavonoids: Carnitine, Choline, Inositol, Taurine, and Ubiquinone Phytoceuticals 5 Priorities for Today’s Session B Vitamins What they are Source Function Requirement Absorption Deficiency Toxicity 6 7 What Is Vitamin B1 First B Vitamin to be discovered 8 Vitamin B1 Sources Pork – rich source Potatoes Whole-grain cereals Meat Fish 9 Functions of Vitamin B1 Converts carbohydrates into glucose for energy metabolism Strengthens immune system Improves body’s ability to withstand stressful conditions 10 Thiamine Requirements Groups: RDA (mg/day): Infants 0.4 Children 0.7-1.2 Males 1.5 Females 1 Pregnancy 2 Lactation 2 11 Thiamine Absorption Absorbed in the duodenum and proximal jejunum Alcoholics are especially susceptible to thiamine deficiency Excreted in urine, diuresis, and sweat Little storage of thiamine in the body 12 Barriers to Thiamine Absorption Lost into cooking water Unstable to light Exposure to sunlight Destroyed -

(12) Patent Application Publication (10) Pub. No.: US 2005/0196469 A1 Thys-Jacobs (43) Pub
US 2005O196469A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2005/0196469 A1 Thys-Jacobs (43) Pub. Date: Sep. 8, 2005 (54) MICRONUTRIENT SUPPLEMENT (22) Filed: Mar. 4, 2004 COMBINATION FOR ACNE TREATMENT AND PREVENTION Publication Classification (76) Inventor: Susan Thys-Jacobs, Larchmont, NY (51) Int. Cl.' ....................... A61 K 31/59; A61 K 31/525; (US) A61K 33/10; A61K 31/19 (52) U.S. Cl. ......................... 424/687; 514/168; 514/251; Correspondence Address: 514/574 GOTTLEB RACKMAN & REISMAN PC 27O MADSON AVENUE 8TH FLOOR (57) ABSTRACT NEW YORK, NY 100160601 A micronutrient Supplement comprising effective amounts (21) Appl. No.: 10/794,729 of calcium, Vitamin D, and folate treats and prevents acne. US 2005/0196469 A1 Sep. 8, 2005 MICRONUTRIENT SUPPLEMENT COMBINATION therapies include benzoyl peroxide which has comedolytic FOR ACNE TREATMENT AND PREVENTION and antibacterial effects, topical antibacterials Such as eryth romycin or clindamycin, azelaic acid, tazaroc, and topical FIELD OF THE INVENTION retinoids. Acne that is resistant to topical treatment requires oral antibiotics or isotretinoin. Indications for isotretinoin 0001. This invention relates to a micronutrient supple include Severe Scarring, acne that is resistant to oral antibi ment in the treatment of acne Vulgaris and inflammation. In otics and acne present for many years that quickly relapses particular, this invention relates to a multi-vitamin and when an oral antibiotic therapy is discontinued. Of note, oral mineral Supplement for improving skin and hair health. isotretinoin is a potent teratogen. Current Standards of acne therapy include the topical descquarnative drugs and antibac BACKGROUND OF THE INVENTION terial agents. -

Tall Man Lettering List REPORT DECEMBER 2013 1
Tall Man Lettering List REPORT DECEMBER 2013 1 TALL MAN LETTERING LIST REPORT WWW.HQSC.GOVT.NZ Published in December 2013 by the Health Quality & Safety Commission. This document is available on the Health Quality & Safety Commission website, www.hqsc.govt.nz ISBN: 978-0-478-38555-7 (online) Citation: Health Quality & Safety Commission. 2013. Tall Man Lettering List Report. Wellington: Health Quality & Safety Commission. Crown copyright ©. This copyright work is licensed under the Creative Commons Attribution-No Derivative Works 3.0 New Zealand licence. In essence, you are free to copy and distribute the work (including other media and formats), as long as you attribute the work to the Health Quality & Safety Commission. The work must not be adapted and other licence terms must be abided. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nd/3.0/nz/ Copyright enquiries If you are in doubt as to whether a proposed use is covered by this licence, please contact: National Medication Safety Programme Team Health Quality & Safety Commission PO Box 25496 Wellington 6146 ACKNOWLEDGEMENTS The Health Quality & Safety Commission acknowledges the following for their assistance in producing the New Zealand Tall Man lettering list: • The Australian Commission on Safety and Quality in Health Care for advice and support in allowing its original work to be either reproduced in whole or altered in part for New Zealand as per its copyright1 • The Medication Safety and Quality Program of Clinical Excellence Commission, New South -

Inhibitory Effects of Some Flavonoids on Thioredoxin Reductase Purified from Chicken Liver ABSTRACT INTRODUCTION
Brazilian Journal of Poultry Science Revista Brasileira de Ciência Avícola Inhibitory Effects of Some Flavonoids on ISSN 1516-635X 2019 / v.21 / n.2 / 001-008 Thioredoxin Reductase Purified from Chicken http://dx.doi.org/10.1590/1806-9061-2018-0982 Liver Original Article Author(s) ABSTRACT Türkoğlu E.AI https://orcid.org/0000-0001-7850-6456 Thioredoxin reductases (TrxRs) are selenocysteine-containing Kuzu MII https://orcid.org/0000-0002-1375-7673 flavoenzymes that reduce Trxin NADPH-dependent manner. In the Ayasan TIII https://orcid.org/0000-0001-7397-6483 view of the direct vital role of TrxR in a wide range of biochemical and IV Inci H https://orcid.org/0000-0002-9791-0435 physiological processes, methods to inhibit this enzyme are clinically Eratak SVV https://orcid.org/0000-0003-3788-8704 important. TrxR has recently emerged as a new candidate in anticancer I Department of Pharmaceutical Biotechnology, drug investigations because of overexpression in tumorous cells. In this Faculty of Pharmacy, University of Health Sciences, Istanbul 34668, Turkey. study, TrxR from chick liver was purified 94.6-fold with a yield of 4.86% II Deparment of Chemistry, Faculty of Science and a specific activity of 0.19 EU/mg. K and V values of TrxR for and Letters, Ağrı İbrahim Çeçen University, Ağrı M max 04100, Turkey. DTNB were calculated as 0.9 mM and 0,03 EU/mL, respectively. Then, III East Mediterranean Agricultural Research Institute, Karatas Road, Adana 01321, Turkey. the effects of the flavonoids hesperidin, naringenin, chlorogenic acid, IV Department of Animal Science, Faculty of ferulic acid, naringin, 3,4-dihydoxybenzoic acid, and ellagic acid on the Agriculture, Bingöl University, Bingöl 12000, Turkey. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

Genomic Insights Into the Uncultured Genus &Lsquo
The ISME Journal (2014) 8, 2463–2477 & 2014 International Society for Microbial Ecology All rights reserved 1751-7362/14 www.nature.com/ismej ORIGINAL ARTICLE Genomic insights into the uncultured genus ‘Candidatus Magnetobacterium’ in the phylum Nitrospirae Wei Lin1,2,7, Aihua Deng3,7, Zhang Wang4, Ying Li2,5, Tingyi Wen3, Long-Fei Wu2,6, Martin Wu4 and Yongxin Pan1,2 1Biogeomagnetism Group, Paleomagnetism and Geochronology Laboratory, Key Laboratory of the Earth’s Deep Interior, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing, China; 2France-China Bio-Mineralization and Nano-Structures Laboratory, Chinese Academy of Sciences, Beijing, China; 3CAS Key Laboratory of Microbial Physiological and Metabolic Engineering, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China; 4Department of Biology, University of Virginia, Charlottesville, VA, USA; 5State Key Laboratory of Agro-Biotechnology and Laboratoire International Associe Franco-Chinois de Bio-Mineralisation et Nano-Structures, College of Biological Sciences, China Agricultural University, Beijing, China and 6Laboratoire de Chimie Bacte´rienne, Aix-Marseille Universite´, CNRS, Marseille Cedex 20, France Magnetotactic bacteria (MTB) of the genus ‘Candidatus Magnetobacterium’ in phylum Nitrospirae are of great interest because of the formation of hundreds of bullet-shaped magnetite magneto- somes in multiple bundles of chains per cell. These bacteria are worldwide distributed in aquatic environments and have important roles in the biogeochemical cycles of iron and sulfur. However, except for a few short genomic fragments, no genome data are available for this ecologically important genus, and little is known about their metabolic capacity owing to the lack of pure cultures. Here we report the first draft genome sequence of 3.42 Mb from an uncultivated strain tentatively named ‘Ca. -
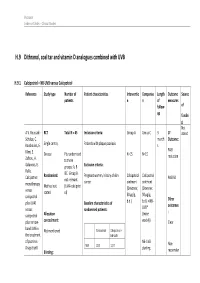
H.9 Dithranol, Coal Tar and Vitamin D Analogues Combined with UVB
Psoriasis Evidence Tables – Clinical Studies H.9 Dithranol, coal tar and vitamin D analogues combined with UVB H.9.1 Calcipotriol + NB-UVB versus Calcipotriol Reference Study type Number of Patient characteristics Interventio Compariso Length Outcome Source patients n n of measures follow- of up fundin g Not A.V. Roussaki- RCT Total N = 45 Inclusion criteria: Group A Group C 3 1º stated Schulze, C. month Outcome: Kouskoukis, E. Single centre, Patients with plaque psoriasis s Klimi, E. PASI Greece Pts randomised N=15 N=15 reduction Zafirou, A. to three Galanous, E. groups: A, B Exclusion criteria: Rallis. &C. Group B Calcipotriol Randomised: Pregnant women, history of skin Calcipotriol Calcipotriol PASI 50 not relevant cancer ointment ointment monotherapy Method not (UVA+calcipotri versus (Dovonex; (Dovonex stated ol) 50 µg/g, 50 µg/g, calcipotriol Other plus UVA1 Baseline characteristics of b.d.) b.d.) + NB- UVB* outcomes versus randomised patients: : calcipotriol Allocation (twice plus narrow- concealment: weekly) Clear band UVB in Calcipotriol Calcipotriol + Not mentioned the treatment NB-UVB of psoriasis. NB-UVB Non- Drugs Exptl. M/F 12/3 12/3 starting Blinding: responder Psoriasis Evidence Tables – Clinical Studies Cl in. Res , dose 80% 31(5/6):169- Not mentioned Age 44.93±6.48 49.53±22.01 MED and 174.2005 inc. by 20% Skin type 0/11/3/1 2/5/6/2 I/II/III/IV every 3 REFID: Washout sessions ROUSSAKISCH period: ULZE2005 90 days if using systemic *Cosmetico therapy, 30 days , 10 lamps if using topicals Helarium B1, 100 W each. -

Nutritional Interventions for Autism Spectrum Disorder
Lead Article Nutritional interventions for autism spectrum disorder Downloaded from https://academic.oup.com/nutritionreviews/advance-article-abstract/doi/10.1093/nutrit/nuz092/5687289 by Florida Atlantic University user on 06 January 2020 Elisa Karhu*, Ryan Zukerman*, Rebecca S. Eshraghi, Jeenu Mittal, Richard C. Deth, Ana M. Castejon, Malav Trivedi, Rahul Mittal, and Adrien A. Eshraghi Autism spectrum disorder (ASD) is an increasingly prevalent neurodevelopmental dis- order with considerable clinical heterogeneity. With no cure for the disorder, treat- ments commonly center around speech and behavioral therapies to improve the characteristic social, behavioral, and communicative symptoms of ASD. Gastrointestinal disturbances are commonly encountered comorbidities that are thought to be not only another symptom of ASD but to also play an active role in modulating the expression of social and behavioral symptoms. Therefore, nutritional interventions are used by a majority of those with ASD both with and without clinical supervision to alleviate gastrointestinal and behavioral symptoms. Despite a consider- able interest in dietary interventions, no consensus exists regarding optimal nutritional therapy. Thus, patients and physicians are left to choose from a myriad of dietary pro- tocols. This review, summarizes the state of the current clinical and experimental liter- ature on nutritional interventions for ASD, including gluten-free and casein-free, keto- genic, and specific carbohydrate diets, as well as probiotics, polyunsaturated fatty -

Enbrace® HR DESCRIPTION: INGREDIENTS
EnBrace® HR with DeltaFolate ™ [1 NF Units] [15 mg DFE Folate] ANTI-ANEMIA PREPARATION as extrinsic/intrinsic factor concentrate plus folate. Prescription Prenatal/Vitamin Drug For Therapeutic Use Multi-phasic Capsules (30ct bottle) NDC 64661-650-30 Rx Only [DRUG] GLUTEN-FREE DESCRIPTION: EnBrace® HR is an orally administered prescription prenatal/vitamin drug for therapeutic use formulated for female macrocytic anemia patients that are in need of treatment, and are under specific direction and monitoring of vitamin B12 and vitamin B9 status by a physician. EnBrace® HR is intended for women of childbearing age who are – or desire to become, pregnant regardless of lactation status. EnBrace® HR may be prescribed for women at risk of depression as a result of folate or cobalamin deficiency - including folate-induced postpartum depression, or are at risk of folate-induced birth defects such as may be found with spina bifida and other neural tube defects (NTDs). INGREDIENTS: Cobalamin intrinsic factor complex 1 NF Units* * National Formulary Units (“NF UNITS”) equivalent to 50 mcg of active coenzyme cobalamin (as cobamamide concentrate with intrinsic factor) ALSO CONTAINS: 1 Folinic acid (B9-vitamer) 2.5 mg + 1 Control-release, citrated folic acid, DHF (B9-Provitamin) 1 mg 2 Levomefolic acid (B9 & B12- cofactor) 5.23 mg 1 6 mg DFE folate (vitamin B9) 2 9 mg DFE l-methylfolate magnesium (molar equivalent). FUNCTIONAL EXCIPIENTS: 13.6 mg FeGC as ferrous glycine cysteinate (1.5 mg 3 3,4 elemental iron ) [colorant], 25 mg ascorbates (24 mg magnesium l-ascorbate, 1 mg zinc l-ascorbate) [antioxidant], at least 23.33 mg phospholipid-omega3 complex5 [marine lipids], 500 mcg betaine (trimethylglycine) [acidifier], 1 mg magnesium l-threonate [stabilizer].