Proposal for Sequencing the Genome of the Tick, Ixodes Scapularis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tick-Borne Diseases Primary Tick-Borne Diseases in the Southeastern U.S
Entomology Insect Information Series Providing Leadership in Environmental Entomology Department of Entomology, Soils, and Plant Sciences • 114 Long Hall • Clemson, SC 29634-0315 • Phone: 864-656-3111 email:[email protected] Tick-borne Diseases Primary tick-borne diseases in the southeastern U.S. Affecting Humans in the Southeastern United Disease (causal organism) Tick vector (Scientific name) States Lyme disease Black-legged or “deer” tick (Borrelia burgdorferi species (Ixodes scapularis) Ticks are external parasites that attach themselves complex) to an animal host to take a blood meal at each of Rocky Mountain spotted fever American dog tick their active life stages. Blood feeding by ticks may (Rickettsia rickettsii) (Dermacentor variabilis) lead to the spread of disease. Several common Southern Tick-Associated Rash Lone star tick species of ticks may vector (transmit) disease. Many Illness or STARI (Borrelia (Amblyomma americanum) tick-borne diseases are successfully treated if lonestari (suspected, not symptoms are recognized early. When the disease is confirmed)) Tick-borne Ehrlichiosis not diagnosed during the early stages of infection, HGA-Human granulocytic Black-legged or “deer” tick treatment can be difficult and chronic symptoms anaplasmosis (Anaplasma (Ixodes scapularis) may develop. The most commonly encountered formerly Ehrlichia ticks in the southeastern U.S. are the American dog phagocytophilum) tick, lone star tick, blacklegged or “deer” tick and HME-Human monocytic Lone star tick brown dog tick. While the brown dog tick is notable Ehrlichiosis (Amblyomma americanum) because of large numbers that may be found indoors (Ehrlichia chafeensis ) American dog tick when dogs are present, it only rarely feeds on (Dermacentor variabilis) humans. -

Ehrlichiosis and Anaplasmosis
Ehrlichiosis and Anaplasmosis The Diseases and Transmission Ehrlichia and Anaplasma are related bacteria that are transmitted by ticks. These bacteria infect white blood cells in humans. There are three different bacteria that cause disease in humans: Anaplasma phagocytophilum Ehrlichia chaffeensis Ehrlichia ewingii Pathogen (formerly Ehrlichia phagocytophila) Human monocytic Human granulocytic Disease Ehrlichiosis ewingii ehrlichiosis (HME) anaplasmosis (HGA, formerly HGE) Tick Vector Amblyomma americanum (lone star tick) Ixodes scapularis (black-legged tick) Animal reservoirs for E. chaffeensis and E. ewingii are white-tailed deer and dogs. The reservoirs for A. phagocytophilum include cattle, deer, and rodents. You cannot get the diseases directly from animals. The diseases are not spread between humans other than through blood transfusions. Maryland is home to both the lone star tick and the black-legged tick. Symptoms and Treatment Disease Clinical Features . Symptoms appear 7 to 10 days after a tick bite. Symptoms include fever, headache, muscle aches, nausea, vomiting, HME, and loss of appetite. Ehrlichiosis ewingii . Meningoencephalitis occurs in approximately 20% of cases. Development of a rash is possible. This may be confused with Rocky Mountain spotted fever. Symptoms appear 7 to 14 days after a tick bite. HGA . Symptoms include fever, headache, and muscle aches. Meningoencephalitis is rare. Most infections occur when tick activity is highest, in late spring and summer. If left untreated, HME and HGA may be severe. Co-infection with more than one tick borne disease is possible. The immune system is directly infected. Secondary infection and other complications can arise quickly. The elderly and sick are more likely to develop severe illness. -

Australiensis Inducing Mammalian Meat Allergy After Tick Bite
Asia Pac Allergy. 2018 Jul;8(3):e31 https://doi.org/10.5415/apallergy.2018.8.e31 pISSN 2233-8276·eISSN 2233-8268 Educational A novel Australian tick Ixodes & Teaching Material Case Report (Endopalpiger) australiensis inducing mammalian meat allergy after tick bite Mackenzie Kwak1, Colin Somerville2, and Sheryl van Nunen 3,4,* 1Department of Biological Science, National University of Singapore, Singapore 2Allergy West, Perth, WA 6149, Australia 3Tick-induced Allergies Research and Awareness Centre, Chatswood, NSW 2067, Australia 4Northern Clinical School, Sydney Medical School, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW 2006, Australia Received: May 11, 2018 Accepted: Jul 25, 2018 ABSTRACT Tick-induced mammalian meat allergy has become an emergent allergy world-wide after *Correspondence to Sheryl van Nunen van Nunen et al. first described the association between tick bites and the development of Tick-induced Allergies Research and mammalian meat allergy in 2007. Cases of mammalian meat allergy have now been reported Awareness Centre, 40 Johnson Street, on all 6 continents where humans are bitten by ticks, in 17 countries - Australia, United Chatswood, NSW 2067, Australia. States of America (USA), Europe (France, Spain, Germany, Belgium, Switzerland, Sweden, Tel: +61411447365 United Kingdom, Italy, and Norway), Asia (Korea and Japan), Central America (Panama), Fax: +6124121694 South America (Brazil), and Africa (South Africa and Ivory Coast). To date, in each of these E-mail: [email protected] countries, bites from only a single tick species have been linked to the development of Copyright © 2018. Asia Pacific Association of mammalian meat allergy: Ixodes holocyclus (Australia), Amblyomma americanum (USA), Ixodes Allergy, Asthma and Clinical Immunology. -
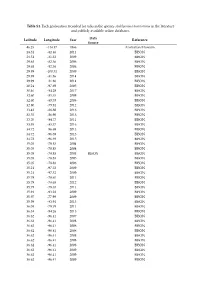
Table S1. Each Geolocation Recorded for Ticks in the Species Amblyomma Americanum in the Literature and Publicly Available Online Databases
Table S1. Each geolocation recorded for ticks in the species Amblyomma americanum in the literature and publicly available online databases. Data Latitude Longitude Year Reference Source 46.25 -114.17 1966 Australian Museum 28.31 -82.46 2011 BISON 28.51 -81.32 2009 BISON 29.68 -82.36 2006 BISON 29.68 -82.36 2006 BISON 29.99 -100.31 2009 BISON 29.99 -81.86 2014 BISON 29.99 -81.86 2014 BISON 30.24 -97.69 2005 BISON 30.46 -84.28 2017 BISON 32.60 -85.35 2008 BISON 32.60 -85.35 2008 BISON 32.80 -79.94 2012 BISON 33.42 -88.88 2016 BISON 33.55 -86.90 2013 BISON 33.70 -84.77 2011 BISON 33.95 -83.37 2016 BISON 34.72 -96.69 2013 BISON 34.72 -96.69 2013 BISON 34.72 -96.69 2015 BISON 35.05 -78.83 2004 BISON 35.05 -78.83 2004 BISON 35.05 -78.83 2004 BISON BISON 35.05 -78.83 2005 BISON 35.05 -78.83 2006 BISON 35.21 -97.32 2009 BISON 35.21 -97.32 2009 BISON 35.79 -78.65 2011 BISON 35.79 -78.65 2012 BISON 35.79 -78.65 2011 BISON 35.91 -93.22 2009 BISON 35.97 -77.99 2009 BISON 35.99 -83.94 2015 BISON 36.08 -79.79 2011 BISON 36.34 -94.26 2015 BISON 36.62 -96.41 2007 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2008 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2009 BISON 36.62 -96.41 2011 BISON 36.62 -96.41 2012 BISON 36.62 -96.41 2012 BISON 37.36 -77.05 2014 BISON 37.62 -84.87 2016 BISON 37.83 -78.28 2011 BISON 37.97 -85.70 2016 BISON 37.97 -85.70 2016 BISON 38.22 -75.31 2014 BISON 38.43 -88.43 -

Mechanisms Affecting the Acquisition, Persistence and Transmission Of
microorganisms Review Mechanisms Affecting the Acquisition, Persistence and Transmission of Francisella tularensis in Ticks Brenden G. Tully and Jason F. Huntley * Department of Medical Microbiology and Immunology, University of Toledo College of Medicine and Life Sciences, Toledo, OH 43614, USA; [email protected] * Correspondence: [email protected] Received: 29 September 2020; Accepted: 21 October 2020; Published: 23 October 2020 Abstract: Over 600,000 vector-borne disease cases were reported in the United States (U.S.) in the past 13 years, of which more than three-quarters were tick-borne diseases. Although Lyme disease accounts for the majority of tick-borne disease cases in the U.S., tularemia cases have been increasing over the past decade, with >220 cases reported yearly. However, when comparing Borrelia burgdorferi (causative agent of Lyme disease) and Francisella tularensis (causative agent of tularemia), the low infectious dose (<10 bacteria), high morbidity and mortality rates, and potential transmission of tularemia by multiple tick vectors have raised national concerns about future tularemia outbreaks. Despite these concerns, little is known about how F. tularensis is acquired by, persists in, or is transmitted by ticks. Moreover, the role of one or more tick vectors in transmitting F. tularensis to humans remains a major question. Finally, virtually no studies have examined how F. tularensis adapts to life in the tick (vs. the mammalian host), how tick endosymbionts affect F. tularensis infections, or whether other factors (e.g., tick immunity) impact the ability of F. tularensis to infect ticks. This review will assess our current understanding of each of these issues and will offer a framework for future studies, which could help us better understand tularemia and other tick-borne diseases. -

Transmission of Cytauxzoon Felis to Domestic Cats by Amblyomma Americanum Nymphs Kelly E
Allen et al. Parasites & Vectors (2019) 12:28 https://doi.org/10.1186/s13071-018-3276-8 RESEARCH Open Access Transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum nymphs Kelly E. Allen*, Jennifer E. Thomas, Megan L. Wohltjen and Mason V. Reichard Abstract Background: Successful Cytauxzoon felis transmission studies have occurred using Amblyomma americanum adults acquisition-fed as nymphs on an experimentally infected domestic cat or Dermacentor variabilis adults fed as nymphs on a splenectomized bobcat. Here, we evaluated A. americanum and D. variabilis nymphs acquisition-fed as larvae on a C. felis-infected carrier domestic cat for competence to transmit the protozoan parasite as nymphs to naïve, healthy domestic cats. Methods: Amblyomma americanum and D. variabilis larvae were applied to a chronically infected, parasitemic C. felis donor cat (Felis catus) and allowed to feed to repletion. Engorged larvae were collected and held through ecdysis. Three cats were each infested with 66 A. americanum or 66 D. variabilis emerged nymphs. Cytauxzoon felis infections in principal cats were determined by clinical signs and detection of circulating parasite by blood smear and PCR evaluation. Results: Clinical signs of cytauxzoonosis were observed in cats infested with A. americanum nymphs beginning 12–15 days post-infestation (dpi). The same cats were PCR positive on 12–14 dpi; piroplasms were evident in blood smears at 16 dpi, and macrophage schizonts were observed in stained spleen impression smears in two animals at necropsy. Cats infested with acquisition-fed D. variabilis nymphs remained clinically normal and did not develop detectable parasitemia over the course of the study as determined by blood smear and PCR. -

Virome Analysis of Amblyomma Americanum, Dermacentor Variabilis, and Ixodes Scapularis Ticks Reveals Novel Highly Divergent Vertebrate and Invertebrate Viruses
Virome Analysis of Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis Ticks Reveals Novel Highly Divergent Vertebrate and Invertebrate Viruses Rafal Tokarz, Simon Hedley Williams, Stephen Sameroff, Maria Sanchez Leon, Komal Jain, W. Ian Lipkin Center for Infection and Immunity, Mailman School of Public Health, Columbia University, New York, New York, USA ABSTRACT A wide range of bacterial pathogens have been identified in ticks, yet the diversity of viruses in ticks is largely unexplored. In the United States, Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis are among the principal tick species associated with pathogen transmission. We used high-throughput sequencing to characterize the viromes of these tick species and identified the presence of Powassan virus and eight novel viruses. These included the most divergent nairovirus described to date, two new clades of tick-borne phleboviruses, a mononegavirus, and viruses with similarity to plant and insect viruses. Our analysis revealed that ticks are reservoirs for a wide range of viruses and suggests that discovery and characterization of tick- borne viruses will have implications for viral taxonomy and may provide insight into tick-transmitted diseases. IMPORTANCE Ticks are implicated as vectors of a wide array of human and animal pathogens. To better understand the extent of tick-borne diseases, it is crucial to uncover the full range of microbial agents associated with ticks. Our current knowledge of the diversity of tick-associated viruses is limited, in part due to the lack of investigation of tick viromes. In this study, we examined the viromes of three tick species from the United States. We found that ticks are hosts to highly divergent viruses across several taxa, includ- ing ones previously associated with human disease. -

Ehrlichia and Spotted Fever Group Rickettsiae Surveillance in Amblyomma Americanum in Virginia Through Use of a Novel Six-Plex Real- Time PCR Assay David N
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Old Dominion University Old Dominion University ODU Digital Commons Biological Sciences Faculty Publications Biological Sciences 2014 Ehrlichia and Spotted Fever Group Rickettsiae Surveillance in Amblyomma americanum in Virginia Through Use of a Novel Six-Plex Real- Time PCR Assay David N. Gaines Darwin J. Operario Suzanne Stroup Ellen Stromdahl Chelsea Wright Old Dominion University See next page for additional authors Follow this and additional works at: https://digitalcommons.odu.edu/biology_fac_pubs Part of the Environmental Public Health Commons, and the Immunology and Infectious Disease Commons Repository Citation Gaines, David N.; Operario, Darwin J.; Stroup, Suzanne; Stromdahl, Ellen; Wright, Chelsea; Gaff, Holly; Broyhill, James; Smith, Joshua; Norris, Douglas E.; Henning, Tyler; Lucas, Agape; and Houpt, Eric, "Ehrlichia and Spotted Fever Group Rickettsiae Surveillance in Amblyomma americanum in Virginia Through Use of a Novel Six-Plex Real-Time PCR Assay" (2014). Biological Sciences Faculty Publications. 299. https://digitalcommons.odu.edu/biology_fac_pubs/299 Original Publication Citation Gaines, D. N., Operario, D. J., Stroup, S., Stromdahl, E., Wright, C., Gaff, H., . Houpt, E. (2014). Ehrlichia and spotted fever group rickettsiae surveillance in Amblyomma americanum in Virginia through use of a novel six-plex real-time PCR assay. Vector-Borne and Zoonotic Diseases, 14(5), 307-316. doi:10.1089/vbz.2013.1509 Authors David N. Gaines, Darwin J. Operario, Suzanne Stroup, Ellen Stromdahl, Chelsea Wright, Holly Gaff, James Broyhill, Joshua Smith, Douglas E. Norris, Tyler Henning, Agape Lucas, and Eric Houpt This article is available at ODU Digital Commons: https://digitalcommons.odu.edu/biology_fac_pubs/299 VECTOR-BORNE AND ZOONOTIC DISEASES Volume 14, Number 5, 2014 ORIGINAL ARTICLES ª Mary Ann Liebert, Inc. -

Chapter 126 – Tickborne Illnesses
CrackCast Show Notes – Tickborne illnesses – December 2017 www.canadiem.org/crackcast Chapter 126 – Tickborne illnesses Episode overview 1) For each of the following illnesses, list the name of the pathogen , the most common tick vector, and the approximate geographic distribution of illness: a. Lyme Disease b. Tularemia c. RMSF d. Q-Fever e. Erlichiosis f. Babesiosis g. Colorado Tick Fever h. Tick Paralysis 2) Describe the difference between the Argasid Ticks and the Ixodid ticks as it relates to disease transmission. Which one tick-borne illness is transmitted by an Argasid tick? 3) Describe the 3 phases of Lyme Disease, and give a strategy for diagnosis. List 4 problems with serology testing in Lyme disease 4) Describe erythema migrans. a. How quickly does it spread? b. List 8 ddx for erythema migrans. 5) Describe the treatment of Early Lyme disease, Early Disseminated Infection and Late Infection 6) What is the Jarisch-Herxheimer reaction? How is it treated? 7) Describe the clinical presentation of tick relapsing fever. How is it treated? 8) What animals are a source of Tularemia? What is the infecting organism? How does it present clinically? How is it treated? 9) Describe the clinical presentation of RMSF. What are 3 non-dermatologic manifestations? How is it diagnosed and treated? 10) What is the weakness pattern of tick paralysis? What is the pathogenesis? How is it treated? Wisecracks 1) How do you remove a tick? 2) What can cause a false positive for lyme disease? 3) What is STARI? Rosen’s In Perspective: None this time! CrackCast -

Emerging Tickborne Diseases
CDC PUBLIC HEALTH GRAND ROUNDS Emerging Tickborne Diseases AAccessible version: https://youtu.be/al5EM3yh--0 March 21, 2017 Expanding Diversity and Distribution of Tickborne Diseases Rebecca Eisen, PhD Research Biologist Division of Vector-Borne Diseases National Center for Emerging and Zoonotic Infectious Diseases The Basics of Tickborne Diseases All known tickborne infectious diseases are diseases of animals that can be transmitted to humans via a tick vector (e.g., zoonoses) ● Ticks can maintain the pathogens through transmission to their offspring ● Ticks can acquire infection through feeding on infectious hosts Humans are incidental hosts, infected by the bite of infected ticks Ticks Can Transmit Diverse Types of Bacteria in the United States Bacterial Diseases (9) Pathogens (14) Tick Genera (5) Anaplasmosis Anaplasma phagocytophilum Ixodes spp. Borrelia miyamotoi disease Borrelia miyamotoi Ixodes spp. Ehrlichia chaffeensis Amblyomma spp. Ehrlichiosis Ehrlichia ewingii Ixodes spp. Ehrlichia muris eauclarensis Borrelia burgdorferi Lyme disease Ixodes spp. Borrelia mayonii Rickettsia parkeri rickettsiosis Rickettsia parkeri Amblyomma spp. Dermacentor spp. Rocky Mountain spotted fever Rickettsia rickettsii Rhipicephalus spp. Pacific Coast tick fever Rickettsia philipii Dermacentor spp. Borrelia hermsii Relapsing fever Borrelia parkeri Ornithodoros spp. Borrelia turicatae Amblyomma spp. Tularemia Francisella tularensis Dermacentor spp. Eisen RJ, Kugeler KJ, Eisen L et al. (2017) ILAR J, in press. Other Types of Pathogens Ticks Can Transmit Diseases (4) Pathogens (4) Tick Genera (3) Viruses Colorado tick fever virus Colorado tick fever Dermacentor spp. (Coltivirus) Heartland virus Heartland virus disease Amblyomma spp. (Phlebovirus) Powassan virus Powassan encephalitis Ixodes spp. (Flavivirus) Protozoa Babesiosis Babesia microti Ixodes spp. Eisen RJ, Kugeler KJ, Eisen L et al. -

The Lone Star Tick, Amblyomma Americanum (Linnaeus) (Acari: Ixodidae) in Maryland
September 2011 The Maryland Entomologist Volume 5, Number 3 An Increasing Presence: The Lone Star Tick, Amblyomma americanum (Linnaeus) (Acari: Ixodidae) in Maryland John F. Carroll United States Department of Agriculture, Agricultural Research Service, Invasive Insect Biocontrol and Behavior Laboratory, Beltsville Agricultural Research Center, Beltsville, Maryland 20705 [email protected] ABSTRACT: The Lone Star Tick, Amblyomma americanum (Linnaeus), has expanded its range in Maryland in recent years. The host-seeking seasonality of the larva, nymph, and adult of A. americanum is summarized for areas of Maryland where this species has invaded since 1990. Flag sampling showed well established populations of A. americanum in western Prince George‟s and Anne Arundel Counties. An indication of the successful establishment of A. americanum in the expansion zone was the presence of dense concentrations of host-seeking nymphs. INTRODUCTION In recent years, the Lone Star Tick, Amblyomma americanum (Linnaeus) (Acari: Ixodidae) (Figure 1, Figure 2), has been expanding it range and becoming more numerous in areas within its existing range (Ginsberg et al. 1991; Ginsberg and Zhioua 1996; Davidson et al. 1994; Means and White 1997; Childs and Paddock 2003; Lindgren et al. 2005; Carroll 2007). The core of the range of A. americanum is the southeastern and south central United States. The range attenuates northward along the Atlantic Coast, with populations on Long Island and Rhode Island (Keirans and Durden 1998). In Iowa, Lindgren et al. (2005) report a northward spread of A. americanum. Lone Star Ticks have long occurred in Maryland, but Carroll (2007) documented a westward spread of this species in the state since at least 1990. -

Feline Tick-Borne Diseases in the United States
PARASITOLOGY EXPERTISE FROM THE NCVP Peer Reviewed Parasitology Expertise from the NCVP FELINE FELINE TICK-BORNE FRIENDLY DISEASES ARTICLE Yoko Nagamori, DVM, and Mason V. Reichard, MS, PhD Oklahoma State University Welcome to one of 3 new columns in this issue, brought to you in partnership between the National Center for Veterinary Parasitology (ncvetp.org) and Today’s Veterinary Practice. The mission of the NCVP is to further the discipline of parasitology by bringing together partners from academia and industry to address emerging issues, serving the veterinary profession by developing future leaders in veterinary parasitology, and providing diagnostic and consulting services worldwide. Its goals include to: • Train graduate veterinarians and other scientists in clinical, applied veterinary parasitology • Promote outstanding, clinically relevant veterinary parasitology research • Provide diagnostic veterinary services in clinical parasitology to practicing veterinarians • Provide balanced, science-based consulting expertise on parasite treatment and prevention strategies. Ticks on domestic cats are often overlooked tick is an important vector for Cytauxzoon as parasites and vectors of disease agents. This felis (cytauxzoonosis),2,3 Francisella tularensis oversight results from the belief that cats are (tularemia),4 and Ehrlichia species (ehrlichiosis) in fastidious groomers and remove these ectoparasites domestic cats.5 before attachment. It is also possible that some cats spend a majority of their time indoors, leading Dermacentor andersoni owners to believe that the cats are not exposed to Dermacentor andersoni, the Rocky Mountain ticks. There are a limited number of studies that wood tick, is distributed throughout the Rocky report feline tick infestations and occurrence of Mountains in the western part of the U.S.