Skeletal Muscle Response to Tenotomy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gluteal Muscle Contracture: Diagnosis and Management Options
SICOT J 2017, 3,1 Ó The Authors, published by EDP Sciences, 2017 DOI: 10.1051/sicotj/2016036 Available online at: www.sicot-j.org REVIEW ARTICLE OPEN ACCESS Gluteal muscle contracture: diagnosis and management options Saroj Rai1, Chunqing Meng1,*, Xiaohong Wang1, Nabin Chaudhary2, Shengyang Jin1, Shuhua Yang1, and Hong Wang1 1 Department of Orthopedics, Wuhan Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, #1277 Jiefang Avenue, 430022 Wuhan, P.R. China 2 Department of Radiology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, #1095 Jiefang Avenue, 430030 Wuhan, P.R. China Received 14 April 2016, Accepted 29 October 2016, Published online 6 January 2017 Abstract – Gluteal muscle contracture (GMC), a debilitating disease, exists all over the globe but it is much more prevalent in China. Patients typically present with abduction and external rotation of the hip and are unable to bring both the knees together while squatting. Multiple etiologies have been postulated, the commonest being repeated intramuscular injection into the buttocks. The disease is diagnosed primarily by clinical features but radiological features are necessary for the exclusion of other pathological conditions. Non-operative treatment with physiotherapy can be tried before surgery is considered but it usually fails. Different surgical techniques have been described and claimed to have a better outcome of one over another but controversy still exists. Based on published literatures, the clinical outcome is exceptionally good in all established methods of surgery. However, endoscopic surgery is superior to conventional open surgery in terms of cosmetic outcome with fewer complications. Nevertheless, its use has been limited by lack of adequate knowledge, instrumentations, and some inherent limitations. -

Cancer Cachexia Decreases Specific Force and Accelerates Fatigue in Limb Muscle
Biochemical and Biophysical Research Communications 435 (2013) 488–492 Contents lists available at SciVerse ScienceDirect Biochemical and Biophysical Research Communications journal homepage: www.elsevier.com/locate/ybbrc Cancer cachexia decreases specific force and accelerates fatigue in limb muscle ⇑ B.M. Roberts a, G.S. Frye b, B. Ahn b, L.F. Ferreira b,1, A.R. Judge a,1, a 1225 Center Drive, HPNP Building Room 1142, Department of Physical Therapy, University of Florida, Gainesville, FL 32610, USA b 1864 Stadium Road, Department of Applied Physiology & Kinesiology, University of Florida, Gainesville, FL 32610, USA article info abstract Article history: Cancer cachexia is a complex metabolic syndrome that is characterized by the loss of skeletal muscle Received 30 April 2013 mass and weakness, which compromises physical function, reduces quality of life, and ultimately can Available online 11 May 2013 lead to mortality. Experimental models of cancer cachexia have recapitulated this skeletal muscle atro- phy and consequent decline in muscle force generating capacity. However, more recently, we provided Keywords: evidence that during severe cancer cachexia muscle weakness in the diaphragm muscle cannot be Muscle weakness entirely accounted for by the muscle atrophy. This indicates that muscle weakness is not just a conse- Muscle atrophy quence of muscle atrophy but that there is also significant contractile dysfunction. The current study C-26 aimed to determine whether contractile dysfunction is also present in limb muscles during severe Contractile dysfunction Colon-26 (C26) carcinoma cachexia by studying the glycolytic extensor digitorum longus (EDL) muscle and the oxidative soleus muscle, which has an activity pattern that more closely resembles the dia- phragm. -

Techniques in Hand & Upper Extremity Surgery
Open Journal of Orthopedics, 2016, 6, 321-325 http://www.scirp.org/journal/ojo ISSN Online: 2164-3016 ISSN Print: 2164-3008 Techniques in Hand & Upper Extremity Surgery Anna De Leo, Billy Ching Leung*, Henk Giele Department of Plastic Surgery, John Radcliffe Hospital, Oxford University Hospital NHS Trust, Oxford, UK How to cite this paper: De Leo, A., Leung, Abstract B.C. and Giele, H. (2016) Techniques in Hand & Upper Extremity Surgery. Open The use of tendon transfer to restore functions of extremities was initially recognised Journal of Orthopedics, 6, 321-325. in the 19th century, and its advancement was further amplified by the polio epidemic http://dx.doi.org/10.4236/ojo.2016.610042 towards the turn of that century. Tendon transfer surgery extended to the use for Received: August 18, 2016 traumatic reconstructive surgery during World War I, with key surgical pioneers, in- Accepted: October 16, 2016 cluding Mayer, Sterling Bunnell, Guy Pulvertaft and Joseph Boyes. In 1921, Robert Published: October 19, 2016 Jones first described the transfer of pronator teres (PT) to the wrist extensors for ir- reparable radial nerve paralysis in infantile hemiplegia. Although, a detailed descrip- Copyright © 2016 by authors and Scientific Research Publishing Inc. tion of its indication and surgical outcomes were not published until 1959 and 1970 This work is licensed under the Creative by Stelling and Meyer, and Keats, respectively. Pronator teres is often the tendon of Commons Attribution International choice for reconstructing wrist extensors, and used in a multiple of pathologies, in- License (CC BY 4.0). cluding radial nerve palsy, cerebral palsy, and tetraplegia. -

ICD~10~PCS Complete Code Set Procedural Coding System Sample
ICD~10~PCS Complete Code Set Procedural Coding System Sample Table.of.Contents Preface....................................................................................00 Mouth and Throat ............................................................................. 00 Introducton...........................................................................00 Gastrointestinal System .................................................................. 00 Hepatobiliary System and Pancreas ........................................... 00 What is ICD-10-PCS? ........................................................................ 00 Endocrine System ............................................................................. 00 ICD-10-PCS Code Structure ........................................................... 00 Skin and Breast .................................................................................. 00 ICD-10-PCS Design ........................................................................... 00 Subcutaneous Tissue and Fascia ................................................. 00 ICD-10-PCS Additional Characteristics ...................................... 00 Muscles ................................................................................................. 00 ICD-10-PCS Applications ................................................................ 00 Tendons ................................................................................................ 00 Understandng.Root.Operatons..........................................00 -

Spinal Muscular Atrophy
FACT SHEET SPINAL MUSCULAR ATROPHY Spinal Muscular Atrophy (SMA) is a Motor Neuron Disease. It is caused by the mutation of the Survival of SYMPTOMS IN INFANTS • Muscle weakness. Motor Neuron (SMN) gene. It occurs due to the loss of • Muscle atrophy (wasting). motor neurons within the spinal cord and brain. It results • Poor muscle tone. in the progressive wasting away of muscles (atrophy) and • Areflexia (delayed reflexes). muscle weakness. SMA can affect people of all ages, races • Weak cry. or genders; however, the majority of cases occur in infancy • Difficulty sucking or swallowing. or childhood. There are four types of SMA. • Feeding difficulties. FORMS OF SMA • Weak cough. • Lack of developmental milestones (inability to lift head TYPE I (ACUTE INFANTILE) or sit up). • Also called Wernig-Hoffman Disease. • Limpness or a tendency to flop. • Most severe form of SMA. • Accumulations of secretions in the lungs or throat. • Usually diagnosed before six months of age. • Those affected cannot sit without support, lungs may SYMPTOMS IN ADULTS not fully develop, swallowing and breathing may be • Muscle weakness. difficult and there is weakness of the intercostal muscles • Muscle atrophy (wasting). (muscles between the ribs). • Weak tongue. • 95 per cent fatal by 18 • Stiffness. • Cramps. TYPE II (CHRONIC INFANTILE) • Fasciculation (twitching). • Usually diagnosed before the age of two, with the • Clumsiness. majority of cases diagnosed by 15 months. • Dyspnea (shortness of breath). • May be able to sit without assistance or even stand with support. DIAGNOSIS • Increased risk for complications from respiratory • A diagnosis can be made by an SMN gene test which infections. -

Skeletal Muscle Damage in COVID-19: a Call for Action
medicina Review Skeletal Muscle Damage in COVID-19: A Call for Action Amira Mohammed Ali 1,2,* and Hiroshi Kunugi 3,4 1 Department of Psychiatric Nursing and Mental Health, Faculty of Nursing, Alexandria University, Alexandria 21527, Egypt 2 Department of Behavioral Medicine, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo 187-8553, Japan 3 Department of Psychiatry, School of Medicine, Teikyo University, Tokyo 173-8605, Japan; [email protected] 4 Department of Mental Disorder Research, National Institute of Neuroscience, National Center of Neurology and Psychiatry, Tokyo 187-8551, Japan * Correspondence: [email protected]; Tel.: +81-042-346-1986 Abstract: Both laboratory investigations and body composition quantification measures (e.g., com- puted tomography, CT) portray muscle loss in symptomatic Coronavirus disease 2019 (COVID-19) patients. Muscle loss is associated with a poor prognosis of the disease. The exact mechanism of muscle damage in COVID-19 patients, as well as the long-term consequences of muscle injury in disease survivors, are unclear. The current review briefly summarizes the literature for mechanisms, assessment measures, and interventions relevant to skeletal muscle insult in COVID-19 patients. Muscle injury is likely to be attributed to the cytokine storm, disease severity, malnutrition, prolonged physical inactivity during intensive care unit (ICU) stays, mechanical ventilation, and myotoxic drugs (e.g., dexamethasone). It has been assessed by imaging and non-imaging techniques (e.g., CT and electromyography), physical performance tests (e.g., six-minute walk test), anthropometric measures (e.g., calf circumference), and biomarkers of muscle dystrophy (e.g., creatine kinase). Interventions Citation: Ali, A.M.; Kunugi, H. -
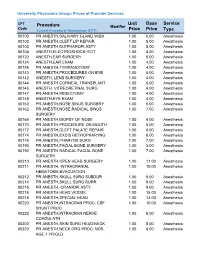
Unit Price Base Price Service Type Procedure
University Physicians Group: Prices of Provider Services CPT Unit Base Service Procedure Modifier Code ype Current Procedural Terminology (CPT) Price Price T 00100 PR ANESTH,SALIVARY GLAND W/BX 1.00 5.00 Anesthesia 00102 PR ANESTH,CLEFT LIP REPAIR 1.00 6.00 Anesthesia 00103 PR ANESTH,BLEPHAROPLASTY 1.00 5.00 Anesthesia 00104 ANESTH,ELECTROSHOCK/ ECT 1.00 4.00 Anesthesia 00120 ANESTH,EAR SURGERY 1.00 5.00 Anesthesia 00124 ANESTH,EAR EXAM 1.00 4.00 Anesthesia 00126 PR ANESTH,TYMPANOTOMY 1.00 4.00 Anesthesia 00140 PR ANESTH,PROCEDURES ON EYE 1.00 5.00 Anesthesia 00142 ANESTH, LENS SURGERY 1.00 4.00 Anesthesia 00144 PR ANESTH,CORNEAL TRANSPLANT 1.00 6.00 Anesthesia 00145 ANESTH, VITREORETINAL SURG 1.00 6.00 Anesthesia 00147 PR ANESTH,IRIDECTOMY 1.00 4.00 Anesthesia 00148 ANESTH,EYE EXAM 1.00 4.00 Anesthesia 00160 PR ANESTH,NOSE,SINUS SURGERY 1.00 5.00 Anesthesia 00162 PR ANESTH,NOSE,RADICAL SINUS 1.00 7.00 Anesthesia SURGERY 00164 PR ANESTH,BIOPSY OF NOSE 1.00 4.00 Anesthesia 00170 PR ANESTH,PROCEDURE ON MOUTH 1.00 5.00 Anesthesia 00172 PR ANESTH,CLEFT PALATE REPAIR 1.00 6.00 Anesthesia 00174 PR ANESTH,EXCIS RETROPHARYNG 1.00 6.00 Anesthesia 00176 PR ANESTH,PHARYNX SURG 1.00 7.00 Anesthesia 00190 PR ANESTH,FACIAL BONE SURGERY 1.00 5.00 Anesthesia 00192 PR ANESTH,RADICAL FACIAL BONE 1.00 7.00 Anesthesia SURGERY 00210 PR ANESTH,OPEN HEAD SURGERY 1.00 11.00 Anesthesia 00211 PR ANESTH, INTRACRANIAL 1.00 10.00 Anesthesia HEMATOMA EVACUATION 00212 PR ANESTH,SKULL SURG SUBDUR 1.00 5.00 Anesthesia 00214 PR ANESTH,SKULL SURG BURR 1.00 9.00 Anesthesia -

Steroid-Induced Myopathy and Its Significance to Respiratory Disease: a Known Disease Rediscovered
Eur Respir J REVIEW 1992, 5, 997-1003 Steroid-induced myopathy and its significance to respiratory disease: a known disease rediscovered P.N.R. Dekhuijzen, M. Decramer Steroid-induced myopathy and its significance to respiratory disease: a known dis Respiratory Muscle Research Unit, ease rediscovered. P.N.R. Dekhuijzen, M. Decramer. Laboratory for Pneumology and Respi ABSTRACT: Skeletal muscle myopathy is a well-known side-effect of systemi ratory Division, University Hospital, cally administered corticosteroids. In recent years renewed attention is being Katholieke Universiteit Leuven, B-3000 Leuvcn, Belgium. paid to the involvement of the respiratory muscles and its consequent signifi cance in pulmonary patients. Two different clinical patterns of steroid-induced Correspondence: M. Decramer, Respira muscular changes are known. In acute myopathy and atrophy after short term tory Division, University Hospital, treatment with high doses of steroids, generalized muscle atrophy and Weligerveld I, 3212 Pellenberg, Bel rhabdomyolysis occur, including the respiratory muscles. Chronic steroid my gium opathy, occurring after prolonged treatment with moderate doses, is character ized by the gradual onset of proximal limb muscle weakness and may be Keywords: Atrophy accompanied by reduced respiratory muscle force. corticosteroids Animal studies demonstrated diaphragmatic myopathy and atrophy similar myopathy respiratory muscles to the alterations in peripheral skeletal muscles. Fluorinated steroids induced selective type lib (fast-twitch -

ACFAS 2020 Poster Presentation
Peroneal Tenodesis and Plantar Fasciotomy for Diabetic Forefoot Ulceration,Case Series Michael D. Liette DPM, Michael J. McGowan DPM, Jordan A. Henning DPM, Bryan J. Hall FACFAS, DPM University of Cincinnati Medical Center, Cincinnati Ohio Statement ofPurpose Procedure Performing a plantar fasciotomy, rather than a posterior Plantar ulceration is common amongst the neuropathic, An incision is made posterior and superior to the lateral heel cord lengthening, may be preferable to reduce the diabetic population. This is due to persistent hyperglycemia, the malleolus, approximately 4 cm in length, isolating and potential creation of a calcaneal gait in a cavus foot. Care production of advanced glycosylation end products, pursuant exposing the peroneal tendons. The peroneus longus tendon must be taken in performing posterior lengthenings in the tendon stiffness with musculature imbalance, and the ultimate is then identified by plantarflexing the first metatarsal and a cavus foot as pseudoequinus may lead to the incorrect effect of increased plantar pressures. Even with the most wedge of the tendon is removed. The proximal portion is diagnosis of equinus and increase the likelihood of the aggressive offloading and local wound care, recurrence rates then sutured to the peroneus brevis tendon under creation of a calcaneal gait and pursuant heel ulceration. remain elevated up to 60%. Deformity is often driven by physiological tension utilizing poly ethylene terephthalate The evidence supporting peroneus longus to peroneus musculature imbalance, eventually producing a compensatory suture. A small incision is then made within the instep of the brevis tenodesis is limited, with no known studies flexible cavus foot with pursuant contracted plantar fascia and medial longitudinal arch, isolating and transecting the evaluating this procedure in isolation to cure or prevent eventual ulceration. -

Resistance Training with Vascular Occlusion in Inclusion Body Myositis: a Case Study
Copyeditor: Jamaica Polintan Resistance Training with Vascular Occlusion in Inclusion Body Myositis: A Case Study BRUNO GUALANO1, MANOEL NEVES JR2, FERNANDA RODRIGUES LIMA2, ANA LU´ CIA DE SA´ PINTO2, GILBERTO LAURENTINO1, CLAUDIA BORGES2, LUCIANA BAPTISTA3, GUILHERME GIANNINI ARTIOLI1, MARCELO SALDANHA AOKI4, ANSELMO MORISCOT4, ANTONIO HERBERT LANCHA JR1, ELOI´SA BONFA´ 2, and CARLOS UGRINOWITSCH1 1School of Physical Education and Sport, University of Sa˜o Paulo, Sa˜o Paulo, BRAZIL; 2Division of Rheumatology, School of Medicine, University of Sa˜o Paulo, Sa˜o Paulo, BRAZIL; 3Hospital Nove de Julho, Sa˜o Paulo, BRAZIL; 4Institute of AQ1 Biomedical Sciences, University of Sa˜o Paulo, Sa˜o Paulo, BRAZIL; and 5School of Arts, Sciences, and Humanities, University of Sa˜o Paulo, Sa˜o Paulo, BRAZIL ABSTRACT GUALANO, B., M. NEVES JR, F. R. LIMA, A. L. PINTO, G. LAURENTINO, C. BORGES, L. BAPTISTA, G. G. ARTIOLI, M. S. AOKI, A. MORISCOT, A. H. LANCHA JR, E. BONFA´ , and C. UGRINOWITSCH. Resistance Training with Vascular Occlusion in Inclusion Body Myositis: A Case Study. Med. Sci. Sports Exerc., Vol. 42, No. 2, pp. 00–00, 2010. Inclusion body myositis (IBM) is a rare idiopathic inflammatory myopathy that produces remarkable muscle weakness. Resistance training with vascular occlusion has been shown to improve muscle strength and cross-sectional area in other muscle wasting conditions. Purpose: We evaluated the efficacy of a moderate-intensity resistance training program combined with vascular occlusion by examining functional capacity, muscle morphology, and changes in the expression of genes related to muscle protein synthesis and proteolysis in a patient with IBM. Methods: A 65-yr-old man with IBM resistant to all proposed treatments underwent resistance training with vascular occlusion for 12 wk. -

If Treatment Fails, Think Inclusion Body Myositis
38 Musculoskeletal Disorders FAMILY P RACTICE N EWS • November 1, 2007 If Treatment Fails, Think Inclusion Body Myositis BY DIANA MAHONEY polymyositis, said Dr. Oddis. “The results fore you get something you can hang your said Dr. Oddis. While no definitive treat- New England Bureau will be abnormal in both conditions, but hat on, but when you do, there is no ques- ment has been proven effective in achiev- MRIs from patients with inclusion body tion,” said Dr. Oddis. “The distinctive his- ing sustained remission and improvement B OSTON — Failure to respond to stan- myositis are more likely to show fatty in- tology that you’re looking for includes en- in whole body strength, some reports sug- dard immunosuppressant therapy may be filtration and atrophy and more widespread domysial inflammation, the presence of gest there may be a subgroup of patients the first sign that a patient’s apparent abnormalities, while in polymyositis, the rimmed vacuoles, and intracellular amy- who experience a partial, transient response polymyositis actually is inclusion body predominant abnormality seen on MRI is loid deposits or twisted tubulofilaments to anti-inflammatory, immunosuppressant myositis, according to Dr. Chester Oddis inflammation distributed along the fascia.” [containing hyperphosphorylated tau].” therapy. For this reason, he said, an initial of the University of Pittsburgh. The only definitive test for inclusion Management options for inclusion body 6-8 week trial of prednisolone and an im- The most common acquired muscle dis- body myositis is a muscle biopsy. Because myositis are often limited to supportive ef- munosuppressive drug such as methotrex- ease in people over 50 years of age, inclu- of the possibility of skip lesions, “it some- forts, such as myotomy to relieve dyspha- ate or azathioprine is a reasonable option sion body myositis (IBM) is a distinct type times takes two, three, or four biopsies be- gia caused by cricopharyngeal achalasia, for newly diagnosed patients. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular