Saururus Cernuus L. Lizard's Tail
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prohibited Species: What NOT to Plant
Prohibited Species: What NOT to Plant e new NJDEP Coastal Management Zone blooms and large red rose hips, this is a non-native Regulations specify that dunes in New Jersey will be plant and should be avoided when restoring native planted with native species only. What follows is a list dunes. Beach plum and bayberry are native plants with of non-indigenous and/or non-native plant species that high habitat value that would be better choices for should NOT be planted on dunes. planting in areas in where rugosa rose is being considered. An alternative rose would be Virginia rose European beachgrass (Ammophila arenaria ) – (Rosa virginiana). While this is not available locally, it is available online and from catalogs for import from California and Salt cedar (Tamarisk sp.) – Its extreme salt tolerance Oregon but would be devastating to the local ecology makes it a common choice for shore gardeners. While if planted here. this plant is not too invasive in dry areas, it is a real threat in riparian areas and planting it should be Dunegrass (Leymus mollis) – is is a native species avoided. to North America but is not indigenous as far south as New Jersey. It occurs from Massachusetts through the Shore juniper (Juniperus conferta) – is species is a Canadian Maritimes and in the Pacific Northwest. non-native, creeping evergreen groundcover and is is plant would not be adapted to our hot, dry used primarily as an ornamental landscaping summers and would not thrive locally. foundation plant. Blue lyme grass (Leymus arenaria) – is is a non- Japanese Black Pine (Pinus thunbergii) – native, ornamental, coastal landscaping grass that is Japanese black pine is highly used by landscapers in not an appropriate plant species for dune stabilization. -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

New Species and Combinations of Apocynaceae, Bignoniaceae, Clethraceae, and Cunoniaceae from the Neotropics
Anales del Jardín Botánico de Madrid 75 (2): e071 https://doi.org/10.3989/ajbm.2499 ISSN: 0211-1322 [email protected], http://rjb.revistas.csic.es/index.php/rjb Copyright: © 2018 CSIC. This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial (by-nc) Spain 4.0 License. New species and combinations of Apocynaceae, Bignoniaceae, Clethraceae, and Cunoniaceae from the Neotropics Juan Francisco Morales 1,2,3 1 Missouri Botanical Garden 4344 Shaw Blvd. St. Louis, MO 63110, USA. 2 Bayreuth Center of Ecology and Environmental Research (BayCEER), University of Bayreuth, Universitätstrasse 30, 95447 Bayreuth, Germany. 3 Doctorado en Ciencias Naturales para el Desarrollo (DOCINADE), Universidad Estatal a Distancia, 474–2050 Montes de Oca, Costa Rica. [email protected], https://orcid.org/0000-0002-8906-8567 Abstract. Mandevilla arenicola J.F.Morales sp. nov. from Brazil, Clethra Resumen. Se describen e ilustran Mandevilla arenicola J.F.Morales secazu J.F.Morales sp. nov. from Costa Rica, and Weinmannia abstrusa sp. nov. de Brasil, Clethra secazu J.F.Morales sp. nov. de Costa Rica y J.F.Morales sp. nov. from Honduras are described and illustrated and Weinmannia abstrusa J.F.Morales sp. nov. de Honduras y se discuten their relationships with morphologically related species are discussed. sus relaciones con otras especies de morfología semejante. Se designan Lectotypes are designated for Anemopaegma tonduzianum Kraenzl., lectotipos para Anemopaegma tonduzianum Kraenzl., Bignonia Bignonia sarmentosa var. hirtella Benth. and Paragonia pyramidata var. sarmentosa var. hirtella Benth. and Paragonia pyramidata var. tomentosa tomentosa Bureau & K. Schum., as well as these last two names have Bureau & K.Schum., así como también se combinan estos dos últimos been combined. -
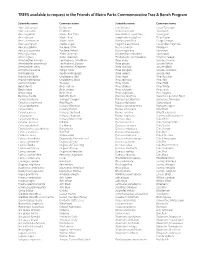
TREES Available to Request in the Friends of Metro Parks Commemorative Tree & Bench Program
TREES available to request in the Friends of Metro Parks Commemorative Tree & Bench Program Scientific name Common name Scientific name Common name Abies balsamaea Fir, Balsam Larix laricina Larch, Tamarack Abies concolor Fir, White Lindera benzoin Spicebush Acer negundo Maple, Box Elder Liquidamber styraciflua Sweetgum Acer rubrum Maple, Red Liriodendron tulipfera Tulip Poplar Acer saccharinum Maple, Silver Maclura pomifera Osage Orange Acer saccharum Maple, Sugar Magnolia acuminata Cucumber magnolia Aesculus glabra Buckeye, Ohio Nyssa sylvatica Blackgum Aesculus octandra Buckeye, Yellow Ostrya viginiana Ironwood Alnus glutinosa Alder, Common Oxydendron arboream Sourwood Alnus rugosa Alder, Speckled Parthenosisis quinquefolia Virginia Creeper Amelanchier arborea Serviceberry, Shadblow Picea abies Spruce, Norway Amelanchier canadensis Serviceberry, Downy Picea glauca Spruce, White Amelanchier laevis Serviceberry Allegheny Picea mariana Spruce, Black Amopha frutocosa Indigo, False Picea pungens Spruce, Colorado Aralia spinosa Devils-walkingstick Picea rubens Spruce, Red Aronia arbutifolia Chokeberry, Red Pinus nigra Pine, Austrian Aronia melancarpa Chokeberry, Black Pinus resinosa Pine, Red Asimina triloba Pawpaw Pinus rigida Pine, Pitch Betula lenta Birch, Yellow Pinus strobus Pine, White Betula lutea Birch, Sweet Pinus sylvestris Pine, Scots Betula nigra Birch, River Pinus virginiana Pine, Virginia Buddeia davidii Butterfly Bush Platanus acerifolia Sycamore, London Plane Campsis radicans Trumpet Creeper Platanus occidentalis Sycamore, -

DESERT WILLOW 'BUBBA' Chilopsis Linearis 'Bubba' Characteristics
DESERT WILLOW ‘BUBBA’ Chilopsis linearis ‘Bubba’ Characteristics Type: Tree Sun: Full sun Zone: 6 to 10 Water: Low to Moderate Height: 25-30 feet Maintenance: Low Spread: 25-30 feet Flower: Showy, Fragrant Bloom Time: Spring through Summer Fruit: Showy Bloom Description: Dark Burgundy and Tolerate: Drought, Dry Soil Pink Texas Native Culture Chilopsis linearis ‘Bubba’ is a vigorous, fast-growing upright selection of desert willow that originated in Texas. Compared to other selections it has a strong vertical form and is less shrubby. It also has glossy, darker green, more lush foliage than other desert willows. It has the capacity to easily grow up to 30 feet tall in the landscape. Starting in late spring and through the summer ‘Bubba’ produces masses of large, fragrant, two-tone burgundy and pink flowers. Like other desert willows ‘Bubba’ is a great choice for full sun, low maintenance, water efficient landscapes. Although it is not seedless, it produces fewer pods than most selections. Each pod containing many winged seeds. Noteworthy Characteristics Chilopsis linearis, commonly known as desert willow, is a large shrub or small multi-trunked tree with a loose open crown. It typically grows to 15-25’ tall with a spread to 10-15’ wide, though some varieties, like ‘Bubba’, grow taller. It is native to gravelly and rocky soils in the Southwestern U.S. and northern Mexico where it is usually found growing in desert grasslands, sandy washes or springs. While the narrow, long leaf shape is indeed willow-like, Chilopsis linearis is in fact related to Catalpa trees, Yellow Bells (Tecoma stans), and Trumpet Vine (Campsis radicans).While oversized in comparison to other members of its family, ‘Bubba’ remains reasonably sized for compact growing spaces as an ornamental accent. -

Native Plants for Your Backyard
U.S. Fish & Wildlife Service Native Plants for Your Backyard Native plants of the Southeastern United States are more diverse in number and kind than in most other countries, prized for their beauty worldwide. Our native plants are an integral part of a healthy ecosystem, providing the energy that sustains our forests and wildlife, including important pollinators and migratory birds. By “growing native” you can help support native wildlife. This helps sustain the natural connections that have developed between plants and animals over thousands of years. Consider turning your lawn into a native garden. You’ll help the local environment and often use less water and spend less time and money maintaining your yard if the plants are properly planted. The plants listed are appealing to many species of wildlife and will look attractive in your yard. To maximize your success with these plants, match the right plants with the right site conditions (soil, pH, sun, and moisture). Check out the resources on the back of this factsheet for assistance or contact your local extension office for soil testing and more information about these plants. Shrubs Trees Vines Wildflowers Grasses American beautyberry Serviceberry Trumpet creeper Bee balm Big bluestem Callicarpa americana Amelanchier arborea Campsis radicans Monarda didyma Andropogon gerardii Sweetshrub Redbud Carolina jasmine Fire pink Little bluestem Calycanthus floridus Cercis canadensis Gelsemium sempervirens Silene virginica Schizachyrium scoparium Blueberry Red buckeye Crossvine Cardinal flower -

Streamhead Canebrakes Are Treeless Or Sparsely Treed Vegetation Dominated by Arundinaria Tecta in Seepage-Fed Drainages
STREAMHEAD CANEBRAKE Concept: Streamhead Canebrakes are treeless or sparsely treed vegetation dominated by Arundinaria tecta in seepage-fed drainages. Tree plus broadleaf shrub cover is generally less than 25 percent in good examples but may be higher if fire frequency has been reduced. Most of this rare community type is in the Sandhills Region, but it might occur in sand dune areas elsewhere in the Coastal Plain. Distinguishing Features: Streamhead Canebrakes are distinguished from other communities of seepage-fed streamheads by the dominance of Arundinaria tecta combined with low cover of trees and other shrubs (less than 25 percent). They are distinguished from Peatland Canebrakes by occurring in streamheads rather than in flat or domed peatlands, Carolina bays, or shallow outer Coastal Plain swales. Synonyms: Arundinaria gigantea ssp. tecta Shrubland (CEGL003843) (not distinguished from Peatland Canebrake in NVC). Atlantic Coastal Plain Streamhead Seepage Swamp, Pocosin and Baygall (CES203.252). Sites: Streamhead Canebrakes occur along mucky headwater and small stream bottoms in dissected sandhill areas, where soils are kept saturated by seepage. Soils: Soils are mucky mineral soils, most often mapped as Johnston (Cumulic Humaquept). Hydrology: Hydrology is typical of the theme as a whole, with long-term saturation by nutrient- poor water but with little or no stream flooding or standing water. Vegetation: Vegetation consists of a dense thicket of Arundinaria tecta and limited cover of broadleaf shrubs. Pinus serotina, Liriodendron tulipifera, Pinus taeda, Nyssa biflora, and Magnolia virginiana may form a sparse canopy. Any of the species of Streamhead Pocosin may be present in moderate numbers. Lyonia lucida is the most abundant other shrub in CVS plot data. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Trumpet Creeper (Campsis Radicans) Control Herbicide Options
Publication 20-86C October 2020 Trumpet Creeper (Campsis radicans) Control Herbicide Options Dr. E. David Dickens, Forest Productivity Professor; Dr. David Clabo, Forest Productivity Professor; and David J. Moorhead, Emeritus Silviculture Professor; UGA Warnell School of Forestry and Natural Resources BRIEF Trumpet creeper (Campsis radicans), also known as cow itch vine, trumpet vine, or hummingbird vine, is in the Bignoniaceae family and is native to the eastern United States. Trumpet creeper is frequently found in a variety of southeastern United States forests and can be a competitor in pine stands. If not controlled, it can kill the trees it grows on by canopying over the crowns and not allowing adequate sunlight to get to the tree’s foliage for photo- synthesis. Trumpet creeper is a deciduous, woody vine that can “climb” trees up to 40 feet or greater heights (Photo 1) or form mats on shrubs or grows in clumps lower to the ground (Photo 2). The 1 to 4 inch long green leaves are pinnate, ovate in shape and opposite (Photo 3). The orange to red showy flowers are terminal cymes of 4 to 10 found on the plants during late spring into summer (Photo 4). Large (3 to 6 inches long) seed pods are formed on mature plants in the fall that hold hundreds of seeds (Photo 5). Trumpet creeper control is best performed during active growth periods from mid-June to early October in Georgia. If trumpet creeper has climbed up into a num- ber of trees, a prescribed burn or cutting the vines to groundline may be needed to get the climbing vine down to groundline where foliar active herbicides will be effective. -

Checklist of Illinois Native Trees
Technical Forestry Bulletin · NRES-102 Checklist of Illinois Native Trees Jay C. Hayek, Extension Forestry Specialist Department of Natural Resources & Environmental Sciences Updated May 2019 This Technical Forestry Bulletin serves as a checklist of Tree species prevalence (Table 2), or commonness, and Illinois native trees, both angiosperms (hardwoods) and gym- county distribution generally follows Iverson et al. (1989) and nosperms (conifers). Nearly every species listed in the fol- Mohlenbrock (2002). Additional sources of data with respect lowing tables† attains tree-sized stature, which is generally to species prevalence and county distribution include Mohlen- defined as having a(i) single stem with a trunk diameter brock and Ladd (1978), INHS (2011), and USDA’s The Plant Da- greater than or equal to 3 inches, measured at 4.5 feet above tabase (2012). ground level, (ii) well-defined crown of foliage, and(iii) total vertical height greater than or equal to 13 feet (Little 1979). Table 2. Species prevalence (Source: Iverson et al. 1989). Based on currently accepted nomenclature and excluding most minor varieties and all nothospecies, or hybrids, there Common — widely distributed with high abundance. are approximately 184± known native trees and tree-sized Occasional — common in localized patches. shrubs found in Illinois (Table 1). Uncommon — localized distribution or sparse. Rare — rarely found and sparse. Nomenclature used throughout this bulletin follows the Integrated Taxonomic Information System —the ITIS data- Basic highlights of this tree checklist include the listing of 29 base utilizes real-time access to the most current and accept- native hawthorns (Crataegus), 21 native oaks (Quercus), 11 ed taxonomy based on scientific consensus. -

Sweetpepper Bush Clethra Alnifolia L
W&M ScholarWorks Reports 11-1-1999 Sweetpepper Bush Clethra alnifolia L. Gene Silberhorn Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/reports Part of the Plant Sciences Commons Recommended Citation Silberhorn, G. (1999) Sweetpepper Bush Clethra alnifolia L.. Wetland Flora Technical Reports, Wetlands Program, Virginia Institute of Marine Science. Virginia Institute of Marine Science, College of William and Mary. http://dx.doi.org/doi:10.21220/m2-ep1m-de63 This Report is brought to you for free and open access by W&M ScholarWorks. It has been accepted for inclusion in Reports by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. Wetlands Technical Report Program Wetland Flora No. 99-11 / November 1999 Gene Silberhorn Sweetpepper Bush Clethra alnifolia L. Growth Habit and Diagnostic Characteristics Habitat Sweetpepper bush is a coastal freshwater shrub with Sweetpepper bush is most likely found in wooded simple, deciduous, alternate leaves (3 to 6 inches wetlands in coastal Virginia, but can grow elsewhere long) and serrated margins. It often grows in dense in non-wetland areas. It is the dominant shrub in the thickets from 3 to 10 feet tall. In mid-summer, the Great Dismal Swamp in Virginia and North Carolina. shrub produces a terminal inflorescence (raceme) of Dense thickets exist there that are difficult to penetrate small, white fragrant flowers. By late summer or early during the growing season. In the Swamp, it is often fall, green, globular capsules (1/8- 1/4 in. wide) associated with fetterbush (Lyonia lucida) and coastal appear (as illustrated) and turn gray by late autumn/ sweetbells (Leucothoe axillaris). -

Mamaki Rust Pucciniastrum Boehmeriae (Dietel) Syd
State of Hawaii New Pest Advisory DEPARTMENT OF AGRICULTURE No. 16-01 May 2016 Mamaki Rust Pucciniastrum boehmeriae (Dietel) Syd. & P. Syd (Pucciniastraceae) Background In August 2013, a diagnostician at the University of Hawaii (UH) Agricultural Diagnostic Service Center, Komohana Research Station incidentally detected an unfamiliar rust on a mamaki (Pipturus albidus) leaf sample from a Hawaiian Acres, Kurtistown residential grower on the Big Island. Consequently, the rust sample was sent to the United States Department of Agriculture, Agricultural Research Service, Systematic Mycology and Microbiology Laboratory (SMML), where it was promptly identified via morphological and molecular means as Pucciniastrum boehmeriae (Dietel) Syd. & P. Syd., a new record in both Hawaii and the U.S. A subsequent visit by the UH diagnostician and Hawaii Department of Agriculture (HDOA) staff to the initial detection site yielded only two more slightly rust infected leaves. Additional surveys at mostly nurseries and botanical gardens throughout the main Hawaiian Islands failed to detect the P. boehmeriae rust. In November 2015, leaf lesions were spotted on wild Boehmeria grandis (akolea) plants in the Southern Koolau Mountains on Oahu by HDOA staff. SMML confirmed the presence of P. boehmeriae on the Oahu akolea leaf samples in February 2016, thus increasing both the known local distribution and susceptible endemic host plant species in the Figure 1. Top view of akolea leaf infected with Pucciniastrum boehmeriae; inset: close - Urticaceae plant family. up. Importance of the Urticaceae in Hawaii Mamaki, akolea, and other related Hawaiian species in the Urticaceae (nettle) family have long been important food sources for various native species of Hawaiian fauna.