Sources and Ages of Carbon and Organic Matter Supporting Macroinvertebrate Production in Temperate Streams
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Upper Susquehanna-Tunkhannock Watershed Text
MAP 33. Pennsylvania Fishing and Boating Access Strategy Upper Susquehanna-Tunkhannock Watershed Text Mitchell Creek ! " ! Wappasening Creek ! Bentley Creek ! ! kj ! 219 !! ! 70 ! ! ! ! !! !! ! Hallstead!! ! Lanesboro! ! Oakland!!" ! 140! 35 Wolcott Creek Seeley Creek " SALT SPRINGS S.P. 123 239 Starrucca kj N ! 175 !! ! B Beaver Creek ! ! r ! ! S ! Thompson Gaylord Cr. ! Mill Creek u Rome ! s ! ! q 11 u ¤£ e " h n kj n Sylvania a !! MOUNT ! R !! PISGAH S.P.!kj . kj Burlington !! 289 Tunkhannock Cr. 236 ! S Wysox Cr. !! Troy ug !! ! ar !" !! Creek "! !! ! " ! Towanda ! Butler Cr. ! !" ! 6 kj" ! ¤£ " Wyalusing Creek ! Meshoppen Creek ! kj !! Monroe " Riley Cr. Hop ! Bottom Alba !"!" !" Wyalusing ! Tioga River ! Towanda Creek !" ! Martins Cr. !! Canton Sugar Run " ! 250 ! "! &É ! Tuscarora Cr. Schrader Cr. 36 Meshoppen Nicholson 36 172 81 ! 12 ! ! ¨¦§ New Lake! " !! kj Carey ! LACKAWANNA S.P. !! Lick Creek Albany 142 ! 220 Tunkhannock Cr. ! Carbondale ¤£ !" !! kj!Lackawanna ! &É Factoryville Lake Dalton Mayfield" ! Tunkhannock 307 " ! ! ! &É Jermyn Kings Creek Dushore !!" ARCHBALD POTHOLE S.P.300 Rock Run ! " ! Elk Creek 11 ! Lick Creek N ! £ " B !! ¤ Archbald 66 r ! Blakely " Forksville S Dickson City " u ! !!" kj s !!kj ! q ! Jessup ! ! ! WORLDS END S.P. Loyalsock Creek Leonard Creek u ! Olyphant ! e ! h ! a n Throop Salt Run n ! ! Mehoopany Creek a Dry Run R Dunmore " Wallis Run . ! ^ 134 57 Scranton^ ! Erie Taylor 84 Bear Creek ! § ! ¨¦ " ! ! !! ! !" kj!!! !! RICKETTS ! Old Forge ! kj Duryea 380 ! GLEN S.P. Bowman Creek Harveys! ! Lake -

SUMMER 2018 Conservation
Northcentral Pennsylvania Conservancy the woods at top speed. A photo has never been forthcoming. Lyons Farm 2018 The barn on the Lyons Farm is the classic style of barn found on small farms in northcentral Pennsylvania, constructed when a farm of 60-100 acres could provide a family with a comfortable way of life. Continued on page 2 Joshi Conservation Projects to Improve Easement Site Visits Local Water By Charlie Schwarz Underway Easement visits almost always The County Conservation Districts entail an opportunity to take photo- in our region, the Pennsylvania Fish & graphs of something interesting, Boat Commission, and Pennsylvania whether it’s a building, relic, plant, Department of Environmental insect, mammal or bird. But, although Protection have been on a Limestone buildings, relics, plants and insects Sustainably conserving the Run, Turtle Creek, Wolf Run, and Indian provide easy – usually – chances for a rural landscapes and waters Creek over the last several weeks. Log photograph, mammals and birds often don’t structures have been installed to cooperate. stabilize stream banks, crossings built for livestock, and trees planted to provide shade over the stream. Joshi 2018 More work will be happening in July, August, and In this small field on the Joshi property I see wild September. turkeys on almost every visit, but they always flee to Roberta Metzer Update – This Montour County landowner worked with the partnership a couple years ago. Since then, they decided to fence their livestock out of the stream and Lyons Farm allow additional stabilization work to happen. REPORT TO THE MEMBERSHIP VOLUME 10 • ISSUE 3 •SUMMER 2018 Conservation.. -

Natural Areas Inventory of Bradford County, Pennsylvania 2005
A NATURAL AREAS INVENTORY OF BRADFORD COUNTY, PENNSYLVANIA 2005 Submitted to: Bradford County Office of Community Planning and Grants Bradford County Planning Commission North Towanda Annex No. 1 RR1 Box 179A Towanda, PA 18848 Prepared by: Pennsylvania Science Office The Nature Conservancy 208 Airport Drive Middletown, Pennsylvania 17057 This project was funded in part by a state grant from the DCNR Wild Resource Conservation Program. Additional support was provided by the Department of Community & Economic Development and the U.S. Fish and Wildlife Service through State Wildlife Grants program grant T-2, administered through the Pennsylvania Game Commission and the Pennsylvania Fish and Boat Commission. ii Site Index by Township SOUTH CREEK # 1 # LITCHFIELD RIDGEBURY 4 WINDHAM # 3 # 7 8 # WELLS ATHENS # 6 WARREN # # 2 # 5 9 10 # # 15 13 11 # 17 SHESHEQUIN # COLUMBIA # # 16 ROME OR WELL SMITHFI ELD ULSTER # SPRINGFIELD 12 # PIKE 19 18 14 # 29 # # 20 WYSOX 30 WEST NORTH # # 21 27 STANDING BURLINGTON BURLINGTON TOWANDA # # 22 TROY STONE # 25 28 STEVENS # ARMENIA HERRICK # 24 # # TOWANDA 34 26 # 31 # GRANVI LLE 48 # # ASYLUM 33 FRANKLIN 35 # 32 55 # # 56 MONROE WYALUSING 23 57 53 TUSCARORA 61 59 58 # LEROY # 37 # # # # 43 36 71 66 # # # # # # # # # 44 67 54 49 # # 52 # # # # 60 62 CANTON OVERTON 39 69 # # # 42 TERRY # # # # 68 41 40 72 63 # ALBANY 47 # # # 45 # 50 46 WILMOT 70 65 # 64 # 51 Site Index by USGS Quadrangle # 1 # 4 GILLETT # 3 # LITCHFIELD 8 # MILLERTON 7 BENTLEY CREEK # 6 # FRIENDSVILLE # 2 SAYRE # WINDHAM 5 LITTLE MEADOWS 9 -

2018 Pennsylvania Summary of Fishing Regulations and Laws PERMITS, MULTI-YEAR LICENSES, BUTTONS
2018PENNSYLVANIA FISHING SUMMARY Summary of Fishing Regulations and Laws 2018 Fishing License BUTTON WHAT’s NeW FOR 2018 l Addition to Panfish Enhancement Waters–page 15 l Changes to Misc. Regulations–page 16 l Changes to Stocked Trout Waters–pages 22-29 www.PaBestFishing.com Multi-Year Fishing Licenses–page 5 18 Southeastern Regular Opening Day 2 TROUT OPENERS Counties March 31 AND April 14 for Trout Statewide www.GoneFishingPa.com Use the following contacts for answers to your questions or better yet, go onlinePFBC to the LOCATION PFBC S/TABLE OF CONTENTS website (www.fishandboat.com) for a wealth of information about fishing and boating. THANK YOU FOR MORE INFORMATION: for the purchase STATE HEADQUARTERS CENTRE REGION OFFICE FISHING LICENSES: 1601 Elmerton Avenue 595 East Rolling Ridge Drive Phone: (877) 707-4085 of your fishing P.O. Box 67000 Bellefonte, PA 16823 Harrisburg, PA 17106-7000 Phone: (814) 359-5110 BOAT REGISTRATION/TITLING: license! Phone: (866) 262-8734 Phone: (717) 705-7800 Hours: 8:00 a.m. – 4:00 p.m. The mission of the Pennsylvania Hours: 8:00 a.m. – 4:00 p.m. Monday through Friday PUBLICATIONS: Fish and Boat Commission is to Monday through Friday BOATING SAFETY Phone: (717) 705-7835 protect, conserve, and enhance the PFBC WEBSITE: Commonwealth’s aquatic resources EDUCATION COURSES FOLLOW US: www.fishandboat.com Phone: (888) 723-4741 and provide fishing and boating www.fishandboat.com/socialmedia opportunities. REGION OFFICES: LAW ENFORCEMENT/EDUCATION Contents Contact Law Enforcement for information about regulations and fishing and boating opportunities. Contact Education for information about fishing and boating programs and boating safety education. -

FFY 2009 Williamsport TIP Highway & Bridge
FFY 2009 Williamsport TIP Highway & Bridge Original US DOT Approval Date: 10/01/2008 Current Date: 06/30/2010 Lycoming MPMS #: 61360 Municipality: Title: Grade Crossing Line Item Route: Section: A/Q Status: Improvement Type: RR High Type Crossing Est. Let Date: Actual Let Date: Geographic Limits: Lycoming County Narrative: Railroad Grade Crossing Reserve Line Item, Lycoming County TIP Program Years ($000) Phase Fund FY 2009 FY 2010 FY 2011 FY 2012 2nd 4 Years 3rd 4 Years CONRRX $48 $0 $52 $54 $0 $0 $48 $0 $52 $54 $0 $0 Total FY 2009-2012 Cost $154 MPMS #: 71068 Municipality: Title: WATS Enhance. Line Item Route: Section: A/Q Status: Improvement Type: Transportation Enhancement Est. Let Date: Actual Let Date: Geographic Limits: Lycoming County Narrative: Transportation Enhancement Reserve Line Item, Lycoming County TIP Program Years ($000) Phase Fund FY 2009 FY 2010 FY 2011 FY 2012 2nd 4 Years 3rd 4 Years CONSTE $0 $0 $320 $336 $0 $0 $0 $0 $320 $336 $0 $0 Total FY 2009-2012 Cost $656 MPMS #: 6081 Municipality: Pine (Twp) Title: T-776 English Run Br#2 Route: Section: A/Q Status: Improvement Type: Bridge Replacement Est. Let Date: 11/10/2005 Actual Let Date: 11/10/2005 Geographic Limits: OVER ENGLISH RUN : PINE TWP : T-776 : Narrative: ENGLISH RUN BR#2 TIP Program Years ($000) Phase Fund FY 2009 FY 2010 FY 2011 FY 2012 2nd 4 Years 3rd 4 Years CONBOO $42 $50 $0 $0 $0 $0 CONLOC $11 $13 $0 $0 $0 $0 $53 $63 $0 $0 $0 $0 Total FY 2009-2012 Cost $116 Page 1 of 42 FFY 2009 Williamsport TIP Highway & Bridge Original US DOT Approval Date: 10/01/2008 Current Date: 06/30/2010 Lycoming MPMS #: 5743 Municipality: Moreland (Twp) Title: T-664 over Ltl. -

Lycoming County
LYCOMING COUNTY START BRIDGE SD MILES PROGRAM IMPROVEMENT TYPE TITLE DESCRIPTION COST PERIOD COUNT COUNT IMPROVED Bridge rehabilitation on State Route 2014 over Lycoming Creek in the City of BASE Bridge Rehabilitation State Route 2014 over Lycoming Creek Williamsport 1 $ 2,100,000 1 0 0 Bridge replacement on PA 973 over the First Fork of Larry's Creek in Mifflin BASE Bridge Replacement PA 973 over the First Fork of Larry's Creek Township and epoxy overlay on PA 973 over Larry's Creek in Mifflin Township 1 $ 1,577,634 2 1 0 BASE Bridge Rehabilitation State Route 2039 over Mill Creek Bridge replacement on State Route 2039 over Mill Creek in Loyalsock Township 1 $ 398,640 1 1 0 Bridge rehabilitation on Township Road 434 over Mosquito Creek in Armstrong BASE Bridge Rehabilitation Township Road 434 over Mosquito Creek Township 3 $ 1,220,000 1 1 0 Bridge truss rehabilitation on State Route 2069 over Little Muncy Creek in BASE Bridge Rehabilitation State Route 2069 over Little Muncy Creek Moreland Township 1 $ 1,000,000 1 1 0 Bridge replacement on PA 87 over Tributary to Loyalsock Creek in Upper Fairfield BASE Bridge Replacement PA 87 over Tributary to Loyalsock Creek Township 3 $ 1,130,000 1 1 0 Bridge replacement on State Route 2001 (Elimsport Road) over Branch of Spring BASE Bridge Replacement State Route 2001 over Branch of Spring Creek #1 Creek in Washington Township 1 $ 1,270,000 1 1 0 BASE Bridge Replacement PA 414 over Upper Pine Bottom Run Bridge replacement on PA 414 over Upper Pine Bottom Run in Cummings Township 2 $ 1,620,000 1 1 -

Wild Trout Waters (Natural Reproduction) - September 2021
Pennsylvania Wild Trout Waters (Natural Reproduction) - September 2021 Length County of Mouth Water Trib To Wild Trout Limits Lower Limit Lat Lower Limit Lon (miles) Adams Birch Run Long Pine Run Reservoir Headwaters to Mouth 39.950279 -77.444443 3.82 Adams Hayes Run East Branch Antietam Creek Headwaters to Mouth 39.815808 -77.458243 2.18 Adams Hosack Run Conococheague Creek Headwaters to Mouth 39.914780 -77.467522 2.90 Adams Knob Run Birch Run Headwaters to Mouth 39.950970 -77.444183 1.82 Adams Latimore Creek Bermudian Creek Headwaters to Mouth 40.003613 -77.061386 7.00 Adams Little Marsh Creek Marsh Creek Headwaters dnst to T-315 39.842220 -77.372780 3.80 Adams Long Pine Run Conococheague Creek Headwaters to Long Pine Run Reservoir 39.942501 -77.455559 2.13 Adams Marsh Creek Out of State Headwaters dnst to SR0030 39.853802 -77.288300 11.12 Adams McDowells Run Carbaugh Run Headwaters to Mouth 39.876610 -77.448990 1.03 Adams Opossum Creek Conewago Creek Headwaters to Mouth 39.931667 -77.185555 12.10 Adams Stillhouse Run Conococheague Creek Headwaters to Mouth 39.915470 -77.467575 1.28 Adams Toms Creek Out of State Headwaters to Miney Branch 39.736532 -77.369041 8.95 Adams UNT to Little Marsh Creek (RM 4.86) Little Marsh Creek Headwaters to Orchard Road 39.876125 -77.384117 1.31 Allegheny Allegheny River Ohio River Headwater dnst to conf Reed Run 41.751389 -78.107498 21.80 Allegheny Kilbuck Run Ohio River Headwaters to UNT at RM 1.25 40.516388 -80.131668 5.17 Allegheny Little Sewickley Creek Ohio River Headwaters to Mouth 40.554253 -80.206802 -
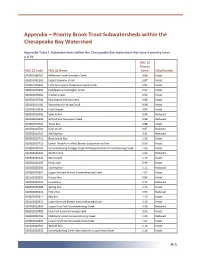
Appendix – Priority Brook Trout Subwatersheds Within the Chesapeake Bay Watershed
Appendix – Priority Brook Trout Subwatersheds within the Chesapeake Bay Watershed Appendix Table I. Subwatersheds within the Chesapeake Bay watershed that have a priority score ≥ 0.79. HUC 12 Priority HUC 12 Code HUC 12 Name Score Classification 020501060202 Millstone Creek-Schrader Creek 0.86 Intact 020501061302 Upper Bowman Creek 0.87 Intact 020501070401 Little Nescopeck Creek-Nescopeck Creek 0.83 Intact 020501070501 Headwaters Huntington Creek 0.97 Intact 020501070502 Kitchen Creek 0.92 Intact 020501070701 East Branch Fishing Creek 0.86 Intact 020501070702 West Branch Fishing Creek 0.98 Intact 020502010504 Cold Stream 0.89 Intact 020502010505 Sixmile Run 0.94 Reduced 020502010602 Gifford Run-Mosquito Creek 0.88 Reduced 020502010702 Trout Run 0.88 Intact 020502010704 Deer Creek 0.87 Reduced 020502010710 Sterling Run 0.91 Reduced 020502010711 Birch Island Run 1.24 Intact 020502010712 Lower Three Runs-West Branch Susquehanna River 0.99 Intact 020502020102 Sinnemahoning Portage Creek-Driftwood Branch Sinnemahoning Creek 1.03 Intact 020502020203 North Creek 1.06 Reduced 020502020204 West Creek 1.19 Intact 020502020205 Hunts Run 0.99 Intact 020502020206 Sterling Run 1.15 Reduced 020502020301 Upper Bennett Branch Sinnemahoning Creek 1.07 Intact 020502020302 Kersey Run 0.84 Intact 020502020303 Laurel Run 0.93 Reduced 020502020306 Spring Run 1.13 Intact 020502020310 Hicks Run 0.94 Reduced 020502020311 Mix Run 1.19 Intact 020502020312 Lower Bennett Branch Sinnemahoning Creek 1.13 Intact 020502020403 Upper First Fork Sinnemahoning Creek 0.96 -

West Branch Subbasin Survey 2015
West Branch Susquehanna River Subbasin Year 1 Survey, April through August 2015 Pub. No.308 September 2016 Ellyn J. Campbell Supervisor, Monitoring and Assessment Susquehanna River Basin Commission TABLE OF CONTENTS INTRODUCTION .......................................................................................................................... 1 METHODS USED IN THE 2015 SUBBASIN SURVEY ............................................................. 5 Long-term Sites ........................................................................................................................... 6 Probabilistic Sites........................................................................................................................ 6 Water Chemistry and Discharge ................................................................................................. 8 Macroinvertebrates ................................................................................................................... 10 Physical Habitat ........................................................................................................................ 11 RESULTS and DISCUSSION ...................................................................................................... 11 CONCLUSIONS........................................................................................................................... 25 REFERENCES ............................................................................................................................. 27 FIGURES -

1 Towanda Creek Basin 01532000 Towanda Creek
1 TOWANDA CREEK BASIN 01532000 TOWANDA CREEK NEAR MONROETON, PA (Pennsylvania Water-Quality Network Station) LOCATION.--Lat 4l°42'25", long 76°29'06", Bradford County, Hydrologic Unit 02050106, on left bank on Township Route 406, 0.8 mi southwest of Monroeton, and 1.0 mi upstream from South Branch Towanda Creek. DRAINAGE AREA.--215 mi2. WATER-DISCHARGE RECORDS PERIOD OF RECORD.--February 1914 to current year. REVISED RECORDS.--WSP 756: Drainage area. WSP 1051: 1943-44(M). WSP 1302: 1922(M), 1924, 1925-26(M), 1928, 1929(M), 1930-31. WSP 1432: 1921(M), 1932(M), 1933, 1934-35(M), 1936, 1938(M), 1940. WDR PA-78-2: 1972(M). WDR PA-87-2: 1978-79. GAGE.--Water-stage recorder. Datum of gage is 765.53 ft above National Geodetic Vertical Datum of 1929. Prior to Oct. 1, 1942, nonrecording gage at present site at datum 8.62 ft higher. Water-stage recorder Oct. 1, 1942, to Sept. 25, 1975, 0.6 mi downstream at datum 11.82 ft lower. Nonrecording gage Sept. 26, 1975, to Aug. 26, 1976, at bridge 0.6 mi downstream at datum 11.82 ft lower. Nonrecording gage Aug. 27, 1976, to Oct. 20, 1977, at present site and datum. REMARKS.--Records good October 1 to July 3; fair, July 4 to September 30, except those for estimated daily discharges, which are poor. Several measurements of water temperature were made during the year. Satellite and landline telemetry at station. PEAK DISCHARGES FOR CURRENT YEAR.--Peak discharges greater than a base discharge of 4,300 ft3/s and maximum (*): Discharge Gage Height Discharge Gage Height Date Time ft3/s (ft) Date Time ft3/s (ft) Mar. -

NOTICES DEPARTMENT of BANKING Actions on Applications
4397 NOTICES DEPARTMENT OF BANKING Actions on Applications The Department of Banking (Department), under the authority contained in the act of November 30, 1965 (P. L. 847, No. 356), known as the Banking Code of 1965; the act of December 14, 1967 (P. L. 746, No. 345), known as the Savings Association Code of 1967; the act of May 15, 1933 (P. L. 565, No. 111), known as the Department of Banking Code; and the act of December 19, 1990 (P. L. 834, No. 198), known as the Credit Union Code, has taken the following action on applications received for the week ending July 20, 2010. Under section 503.E of the Department of Banking Code (71 P. S. § 733-503.E), any person wishing to comment on the following applications, with the exception of branch applications, may file their comments in writing with the Department of Banking, Corporate Applications Division, 17 North Second Street, Suite 1300, Harrisburg, PA 17101-2290. Comments must be received no later than 30 days from the date notice regarding receipt of the application is published in the Pennsylvania Bulletin. The nonconfidential portions of the applications are on file at the Department and are available for public inspection, by appointment only, during regular business hours. To schedule an appointment, contact the Corporate Applications Division at (717) 783-2253. Photocopies of the nonconfidential portions of the applications may be requested consistent with the Department’s Right-to-Know Law Records Request policy. BANKING INSTITUTIONS Branch Applications De Novo Branches Date Name -

Muncy Creek Planning Area 1 Final Draft – February 2003 Technical Background Studies No
TThhee CCoommpprreehheennssiivvee PPllaann BBaacckkggrroouunndd SSttuuddiieess ffoorr tthhee MMuunnccyy CCrreeeekk PPllaannnniinngg AArreeaa Hughesville Borough, Muncy Borough, Muncy Creek Township, Picture Rocks Borough, Shrewsbury Township, Wolf Township Lycoming County, PA Technical Background Studies No. 1 – Community Development Profile Introduction The development of an effective comprehensive plan requires an understanding of the issues and trends that impact a community’s ability to sustain a “good quality of life” for its residents. During the early stages of plan development, coordination has been undertaken with many individuals and organizations in order to develop an understanding of what are perceived to be important issues that will impact the community and its development and growth in the future. This Community Development Profile summarizes where the community has been, where it is today, and where it may be going in the future based on known data sources. It includes past trend information (historic), current trend information (today), and projections (future), where appropriate and available from existing data sources. Key Community Development Issues Through consultation with the Planning Advisory Team (PAT) and interviews with key persons within the planning area and throughout the county, the important issues that could potentially impact the community in terms of social and economic conditions were identified. Issues of importance that relate to the county or region are shown in the adjacent highlight box. Input from the Muncy Creek Planning Advisory Team reflected the rural nature of the area. The overriding important social issue identified by the Planning Advisory Team was the “Small town character of the close knit communities where you know the people.” Thus, one would expect the following issues to be of particular interest in this planning area.