A Lunar Regolith Analogue Study Richard Matthewman , Ri
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Science Organising Committee Local Organising Committee Katherine Joy (Chair) Romain Tartese Mahesh Anand John Pernet-Fisher
Science Organising Committee Local Organising Committee Katherine Joy (Chair) Romain Tartese (Chair) Romain Tartese Katherine Joy Mahesh Anand Patricia Clay John Pernet-Fisher Samantha Bell Kerri Donaldson-Hanna Vera Fernandes Evelyn Furi John Pernet-Fisher Jessica Flahaut Sarah Crowther Greg Schmitt Gemma Coleman James Carpenter Updated: 27 March 2019 European Lunar Symposium Manchester 2019 Meeting information Welcome you to Manchester for the 7th European Lunar Symposium (ELS). We are hoping to have a great meeting, demonstrating the diversity of the current lunar research in Europe and elsewhere, and continuing to provide a platform to the European lunar researchers for networking as well as exchanging news ideas and latest results in the field of lunar exploration. We gratefully acknowledge the support of the University of Manchester, NASA SSERVI, the Royal Astronomical Society, the Science and Technology Facilities Council, Europlanet, and the European Space Agency. Our special thanks to our SSERVI colleagues Kristina Gibbs, Jennifer Baer, Maria Leus, and Ashcon Nejad, and to Gemma Coleman at the University of Manchester for their contribution to the meeting preparation and program implementation. Members of the Science Organising Committee are thanked for their input in putting together an exciting program and for volunteering to chair various sessions in this meeting. Meeting Venue Please note that there are two different venues: • The reception event on the 20 th May will be held at the Manchester Museum in the south of the city, close to the University of Manchester. • The symposium on 21 st -23 rd May will be held at the Science and Industry Museum – Garratt suite conference facilities. -

Exploring the Bombardment History of the Moon
EXPLORING THE BOMBARDMENT HISTORY OF THE MOON Community White Paper to the Planetary Science and Astrobiology Decadal Survey 2023-2032 Sanctioned by the NASA Lunar Exploration Analysis Group July 15, 2020 Author: William F. Bottke Endorsers: Endorser Affiliation Joseph M. Boyce University of Hawaii Ian Crawford Birkbeck College, University of London, UK Qing-Zhu Yin University of California at Davis, Department of Earth and Planetary Sciences Kip Hodges Arizona State University Caitlin Ahrens University of Arkansas Stephen Elardo Department of Geological Sciences, University of Florida Kris Zacny Honeybee Robotics Daoru Han Missouri University of Science and Technology Marc Norman Australian National University Gordon Osinski University of Western Ontario Amy Mainzer University of Arizona J. R. Szalay Princeton University Shashwat Shukla University of Twente, The Netherlands Jonti Horner University of Southern Queensland Audrey Bouvier Universität Bayreuth 1 Katherine Joy The University of Manchester, UK Ross W. K. Potter Brown University Barbara Cohen NASA Goddard Space Flight Center Heather Meyer JHU APL Luke Dones Southwest Research Institute Timothy D. Swindle University of Arizona Kirby D. Runyon Johns Hopkins APL Ross A. Beyer SETI Institute and NASA Ames Research Center Stephen Mojzsis University of Colorado at Boulder Simone Marchi SwRI Dimitri A. Papanastassiou Geol. Planet. Sci., Caltech Christian Tai Udovicic Northern Arizona University Nicolle Zellner Albion College Michelle Kirchoff Southwest Research Institute Meng-Hua Zhu -

BAS Science Summaries 2018-2019 Antarctic Field Season
BAS Science Summaries 2018-2019 Antarctic field season BAS Science Summaries 2018-2019 Antarctic field season Introduction This booklet contains the project summaries of field, station and ship-based science that the British Antarctic Survey (BAS) is supporting during the forthcoming 2018/19 Antarctic field season. I think it demonstrates once again the breadth and scale of the science that BAS undertakes and supports. For more detailed information about individual projects please contact the Principal Investigators. There is no doubt that 2018/19 is another challenging field season, and it’s one in which the key focus is on the West Antarctic Ice Sheet (WAIS) and how this has changed in the past, and may change in the future. Three projects, all logistically big in their scale, are BEAMISH, Thwaites and WACSWAIN. They will advance our understanding of the fragility and complexity of the WAIS and how the ice sheets are responding to environmental change, and contributing to global sea-level rise. Please note that only the PIs and field personnel have been listed in this document. PIs appear in capitals and in brackets if they are not present on site, and Field Guides are indicated with an asterisk. Non-BAS personnel are shown in blue. A full list of non-BAS personnel and their affiliated organisations is shown in the Appendix. My thanks to the authors for their contributions, to MAGIC for the field sites map, and to Elaine Fitzcharles and Ali Massey for collating all the material together. Thanks also to members of the Communications Team for the editing and production of this handy summary. -

Impactor Material in New Lunar Meteorite NWA 10989
Open Research Online The Open University’s repository of research publications and other research outputs Impactor material in new lunar meteorite NWA 10989 Conference or Workshop Item How to cite: Morland, Zoe and Joy, Katherine (2018). Impactor material in new lunar meteorite NWA 10989. In: EPSC Abstracts, 12, article no. 1248. For guidance on citations see FAQs. c 2018 The Authors https://creativecommons.org/licenses/by-nc-nd/4.0/ Version: Version of Record Link(s) to article on publisher’s website: http://adsabs.harvard.edu/abs/2018EPSC...12.1248M Copyright and Moral Rights for the articles on this site are retained by the individual authors and/or other copyright owners. For more information on Open Research Online’s data policy on reuse of materials please consult the policies page. oro.open.ac.uk EPSC Abstracts Vol. 12, EPSC2018-1248, 2018 European Planetary Science Congress 2018 EEuropeaPn PlanetarSy Science CCongress c Author(s) 2018 Impactor material in new lunar meteorite NWA 10989 Zoe Morland, Katherine Joy School of Earth and Environmental Sciences, University of Manchester, Williamson Building, Oxford Road, Manchester, M13 9PL, UK. ([email protected], [email protected]) 1. Introduction scattered electron (BSE) and whole sample BSE merger images, qualitative compositional energy- Lunar meteorites derive from material ejected at dispersive X-ray spectroscopy point spectra and escape velocity from impact events on the Moon. The whole sample elemental map; Raman spectrometer to ejected material is subsequently caught by Earth’s identify cohenite and goethite; and electron probe gravitational field and falls as a meteorite [1]. -

Dispelling the Myth of Robotic Efficiency: Why Human Space
CRAWFORD: HUMAN SPACE EXPLORATION CRAWFORD: HUMAN SPACE EXPLORATION Dispelling the myth of robotic efficiency Ian Crawford explains why human space exploration will tell us more about the solar system than robotic exploration alone. here is a widely held view in the astro- nomical community that unmanned Trobotic space vehicles are, and always will be, more efficient explorers of planetary surfaces than astronauts (e.g. Coates 2001, Cle- ments 2009, Rees 2011). Partly this comes from a common assumption that robotic exploration is cheaper than human exploration (although this isn’t necessarily true if like is compared with like) and partly from the expectation that devel- 1: The increasing size of Mars rovers, from Pathfinder (front left), a Mars Exploration Rover (left), opments in technology will relentlessly increase to Mars Science Laboratory (right). This increase in size (and cost), contrary to predictions that the capability and reduce the size and cost of improved technology will result in smaller and cheaper robots, is mandated by the nature of the robotic missions to the point that human explo- martian surface and complexity of exploration objectives. Human missions would be even larger and ration will not be able to compete. I argue below more expensive, but, crucially, much more capable. (NASA/JPL-Caltech) that the experience of human exploration during the Apollo missions, more recent field analogue 0.32 kg from the Russian robotic sample return for the Mars Exploration Rovers (MERs) Spirit studies and trends in robotic space exploration missions Lunas 16, 20 and 24, and the zero kg and Opportunity, has written: all point to exactly the opposite conclusion. -

The Scientific Legacy of Apollo
The Scientific Legacy of Apollo Ian A. Crawford, Department of Earth and Planetary Sciences, Birkbeck College, University of London ([email protected]). Article published in the December 2012 issue of the Royal Astronomical Society’s journal Astronomy and Geophysics (Vol. 53, pp. 6.24-6.28). Abstract On the 40th anniversary of the last human expedition to the Moon, I review the scientific legacy of the Apollo programme and argue that science would benefit from a human return to the Moon. Introduction This December marks 40 years since the last human beings to set foot on the Moon, Gene Cernan and Harrison “Jack” Schmitt of Apollo 17, left the lunar surface and returned safely to Earth. This anniversary alone would have justified a retrospective look at the legacy of the Apollo project, but it has been given additional poignancy by the death earlier this year of Neil Armstrong, the first man to set foot on the lunar surface with Apollo 11 in July 1969. The history of the Apollo project, and its geopolitical motivation within the context of the Cold War, is of course well documented (e.g. Chaiken 1994; Burrows 1998; Orloff and Harland 2006) and need not be repeated here. However, although the scientific legacy of Apollo has also been well-documented (e.g. Heiken et al. 1991; Wilhelms 1993; Beattie 2001), and is generally well-known within the lunar science community, I have found that it is still underappreciated by many astronomers, and even some planetary scientists who are not directly involved in lunar studies. -

MOON551R1 Title: Lunar Palaeoregolith
Editorial Manager(tm) for Earth, Moon, and Planets Accepted Manuscript Manuscript Number: MOON551R1 Title: Lunar Palaeoregolith Deposits as Recorders of the Galactic Environment of the Solar System and Implications for Astrobiology Article Type: SI:Astrobiology on the Moon Keywords: Moon; Lunar regolith; Galactic history; Galactic structure; Lunar exploration; Astrobiology Corresponding Author: Dr. Ian Crawford, Corresponding Author's Institution: Birkbeck College First Author: Ian Crawford Order of Authors: Ian Crawford; Sarah Fagents; Katherine Joy; Elise Rumpf Manuscript Region of Origin: UNITED KINGDOM Lunar Palaeoregolith Deposits as Recorders of the Galactic Environment of the Solar System and Implications for Astrobiology Ian A. Crawford1,2, Sarah A. Fagents3, Katherine H. Joy2,4,5 3 and M. Elise Rumpf 1Department of Earth and Planetary Sciences, Birkbeck College, University of London, Malet Street, London, WC1E 7HX, UK. Email: [email protected] 2Centre for Planetary Sciences at UCL/Birkbeck, University College London, Gower Street, London, WC1E 6BT. UK. 3Hawaii Institute of Geophysics and Planetology, University of Hawaii, Honolulu, HI 96822, USA. 4The Center for Lunar Science and Exploration, Lunar and Planetary Institute, Houston, TX 77058, USA. 5The NASA Lunar Science Institute. Abstract. One of the principal scientific reasons for wanting to resume in situ exploration of the lunar surface is to gain access to the record it contains of early Solar System history. Part of this record will pertain to the galactic environment of the Solar System, including variations in the cosmic ray flux, energetic galactic events (e.g, supernovae and/or gamma-ray bursts), and passages of the Solar System through dense interstellar clouds. -

From the President
Supplement to Meteoritics & Planetary Science, vol. 52, no. 11 The Meteoritical Society Newsletter (November 2017) A report of the business carried out by The Society over the past year, edited by Michael K. Weisberg, Secretary. IN THIS ISSUE From the President Important reminders Annual meetings Please renew your membership before Dec From the Treasurer 15 as the society has to pay the costs of From the Endowment Committee mailing late reminders. Members renewing Publications reports after March 31 incur a $15 surcharge and MAPS GCA risk missing issues of MAPS. You can renew Elements online at From the Nomenclature Committee http://metsoc.meteoriticalsociety.net. From the Membership Committee Nominate your colleagues and students for Awards and honors awards. Nominations for Fellows will be Call for nominations New Fellows to be elected considered this year. Deadlines are in From the Secretary January. See the Awards section for details. Election of new Council Proposals to host the 2022 MetSoc meeting are due in March. Please contact the secretary for procedures. FROM THE PRESIDENT President’s Report membership, and increasingly the commitment of an individual to take ownership of running our Meeting. The annual newsletter provides the President an I can say from experience that this is a somewhat opportunity to reflect on matters of interest to Society daunting task, but for those of us who have done it, it Members. First off I would like to thank Past has been a complete privilege and the memories President Mike Zolensky for all his hard work in generated go far beyond science, but are more akin to keeping the Society moving forward. -
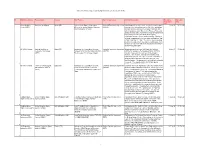
Active International Agreements by Signature Date (As of June 30, 2019)
Active International Agreements by Signature Date (as of June 30, 2019) No. NASA Installation Partner Name Country Title/Purpose Type of Agreement Activity Description Execution Expiration (Signature) Date Date Kennedy Space Government of Spain Spain (SP) Agreement on Space Cooperation Umbrella/Framework Agreement Authorization for, in case of an emergency, manned space 11-Jul-91 31-Dec-00 Center (KSC) Between the United States of America (UM/FW) vehicles of the United States to overfly, enter, and depart and the Kingdom of Spain Spanish air space and use the runways, taxiways, and other installations at the Moron de la Frontera, Rota, and Zaragoza bases; also, agreement to negotiate agreements in promising areas for joint efforts to strengthen cooperation in space science and technology. Dip notes entering the agreement into force were exchange on Sept 3, 1991, and May 12, 1994. The science and technology portion of this agreement was implemented by agreement SP0027 of 12/02/1991 with INTA and agreement SP0028 of 07/03/1992 with CDTI. 1 All NASA Centers National Institute for Spain (SP) Agreement on Cooperative Activities Umbrella/Framework Agreement Broad agreement between NASA and the National 2-Dec-91 31-Dec-00 Aerospace Technology Between NASA and the National Institute (UM/FW) Institute for Aerospace Technology of Spain (INTA) to (INTA) For Aerospace Technology of Spain consider cooperation in a variety of fields in Space Science, Earth Science, Aeronautics Research, and Exploration Systems. The agreement also establishes a group to discuss potential cooperative projects in the identified areas. The agreement is automatically extended each year. -

Scientific Priorities for Robotic and Human Lunar Exploration
AN INTEGRATED SCIENTIFIC AND SOCIAL CASE FOR HUMAN SPACE EXPLORATION A White Paper submitted to the US National Academies Committee on Human Spaceflight by Ian A. Crawford Professor of Planetary Science and Astrobiology Department of Earth and Planetary Sciences, Birkbeck College, University of London Malet Street, London, WC1E 7HX, UK Email: [email protected] Co-Authors (listed alphabetically) Ms Louise Alexander, Birkbeck College, UK Dr Mahesh Anand, Open University, UK Dr Sebastien Besse, European Space Agency (ESTEC), The Netherlands Dr Neil Bowles, University of Oxford, UK Dr Roberto Bugiolacchi, Birkbeck College, UK Professor Mark Burchell, University of Kent, UK Ms Abigail Calzada, Birkbeck College, UK Professor Charles Cockell, University of Edinburgh, UK Professor Hilary Downes, Birkbeck College, UK Dr Sylvie Espinasse, European Space Agency (ESTEC), The Netherlands Professor Heino Falcke, Radboud University, The Netherlands Dr Kevin Fong, University College London, UK Dr Peter Grindrod, University College London, UK Ms Huma Irfan, Birkbeck College, UK Professor Ralf Jaumann, DLR, Institute of Planetary Research, Germany Dr Katherine Joy, University of Manchester, UK Dr Marc Klein-Wolt, Radboud University, The Netherlands Abstract Human spaceflight has the potential to advance human knowledge in a number of areas simultaneously, including materials science, life sciences (including human physiology and medicine), astronomy, and planetary science. Moreover, the benefits of human space exploration extend to a wide array of potential economic, industrial, political, educational, and cultural benefits. These benefits will be especially strong within the context of a global space exploration programme such as envisaged by the Global Exploration Strategy. 1. Introduction Human space exploration has the potential to confer a wide range of scientific, technological, economic, political, and cultural benefits on society. -

PSRD: Searching for Ancient Solar System Materials on the Moon, Earth, and Mars
PSRD: Searching for Ancient Solar System Materials on the Moon, Earth, and Mars November 11, 2016 Searching for Ancient Solar System Materials on the Moon, Earth, and Mars --- The early history of the Solar System is recorded by meteorites falling now, but also by those that fell hundreds of millions to billions of years ago, preserved in lunar samples, sedimentary layers on Earth, and even sitting on the surface of Mars. Written by G. Jeffrey Taylor Hawai'i Institute of Geophysics and Planetology The Moon is a proven collector of rambling objects from throughout the Solar System, as summarized in a review article by Katherine Joy (University of Manchester, England) and colleagues at the University of London, the Lunar and Planetary Institute (Houston), NASA Johnson Space Center, and Western Ontario University. Their article summarizes the record of small bodies impacting the Moon. The lunar record includes chemical signatures in rocks formed by large impacts early in lunar history and rare, hard-to-find fragments of the impactors. Other studies have shown that layers of old sedimentary rocks on Earth also contain fragments of the types of meteorites falling to Earth today. Birger Schmitz (Lund University, Sweden) and coworkers at the University of Hawai‘i, and the University of California, Davis have found a record of in-falling L-chondrites in limestone deposits formed during the Ordovician period (around 500 million years ago). Consistent with meteorite studies, these fossil meteorites indicate that the L-chondrite parent asteroid was demolished by the impact of a chemically different type of asteroid, which sent a plethora of objects into the inner Solar System to intersect Earth hundreds of millions of years ago. -

Iron Meteorites Just Below the Icy Surface
Iron Meteorites Just Below the Icy Surface Quick Views of Big Advances Iron Meteorites Just Below the Icy Surface The continent of Antarctica holds special meaning to meteoriticists. The blue ice fields of the East Antarctic icesheet, for example, have concentrations of meteorites that are the target of recovery expeditions by the US-led Antarctic Search for Meteorites program (ANSMET). See the ANSMET FAQs for a good overview of the why, how, where, and who of the program. ANSMET field teams traverse by snowmobiles or boots to find and recover the meteorites that have been left in lag deposits on the ice surfaces after a long, natural process of ice flow, ice stagnation, and wind ablation/deflation. These concentrations are called meteorite stranding zones. Interestingly, collection statistics show that fewer and smaller masses of iron and stony-iron meteorites are recovered These two pieces of iron meteorite were collected by the 2001-2002 from Antarctica stranding zones than ANSMET team off a meteorite stranding zone in Meteorite Hills, from other places on Earth. What is the Antarctica. [ Data link from the Meteoritical Bulletin. ] reason for this iron deficiency in the Antarctic meteorite collection? According to a team of researchers from the University of Manchester, UK it's a matter of solar radiation, thermal conductivity, thawing, freezing, and sinking. Geoffrey Evatt, M. J. Coughlan, Katherine Joy, Andrew Smedley, Paul Connolly, and David Abrahams combined laboratory experiments with mathematical models to relate their findings to Antarctic conditions. Their results suggest meteorites, a few tens of centimeters below the ice surface, with high-enough thermal conductivity (e.g., those containing iron) will warm and sink at a rate that offsets the local, annual upward ice flow and will remain trapped within the ice...out of sight of meteorite searchers.