Fetal Cerebral Ventriculomegaly
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meninges Ventricles And
Meninges ,ventricles & CSF Dr.Sanaa Al-Shaarawy Dr. Essam Eldin Salama OBJECTIVES • By the end of the lecture the student should be able to: • Describe the cerebral meninges & list the main dural folds. • Describe the spinal meninges & locate the level of the termination of each of them. • Describe the importance of the subarachnoid space. • List the Ventricular system of the CNS and locate the site of each of them. • Describe the formation, circulation, drainage, and functions of the CSF. • Know some clinical point about the CSF MENINGES • The brain and spinal cord are invested by three concentric membranes ; • The outermost layer is the dura matter. • The middle layer is the arachnoid matter. • The innermost layer is the pia matter. DURA MATER ▪The cranial dura is a two layered tough, fibrous thick membrane that surrounds the brain. ▪It is formed of two layers; periosteal and meningeal. ▪The periosteal layer is attached to the skull. ▪The meningeal layer is folded forming the dural folds : falx cerebri, and tentorium cerebelli. ▪Sensory innervation of the dura is mostly from : meningeal branches of the trigeminal and vagus nerves & C1 to C3(upper cervical Ns.). DURA MATER Folds Two large reflection of dura extend into the cranial cavity : 1.The falx cerebri, In the midline, ▪It is a vertical sickle-shaped sheet of dura, extends from the cranial roof into the great longitudinal fissure between the two cerebral hemispheres. ▪It has an attached border adherent to the skull. ▪And a free border lies above the corpus callosum. DURA MATER Folds 2. A horizontal shelf of dura, The tentorium cerebelli, ▪ It lies between the posterior part of the cerebral hemispheres and the cerebellum. -

A Recommended Workflow Methodology in the Creation of An
Manson et al. BMC Medical Imaging (2015) 15:44 DOI 10.1186/s12880-015-0088-6 TECHNICAL ADVANCE Open Access A recommended workflow methodology in the creation of an educational and training application incorporating a digital reconstruction of the cerebral ventricular system and cerebrospinal fluid circulation to aid anatomical understanding Amy Manson1,2, Matthieu Poyade2 and Paul Rea1* Abstract Background: The use of computer-aided learning in education can be advantageous, especially when interactive three-dimensional (3D) models are used to aid learning of complex 3D structures. The anatomy of the ventricular system of the brain is difficult to fully understand as it is seldom seen in 3D, as is the flow of cerebrospinal fluid (CSF). This article outlines a workflow for the creation of an interactive training tool for the cerebral ventricular system, an educationally challenging area of anatomy. This outline is based on the use of widely available computer software packages. Methods: Using MR images of the cerebral ventricular system and several widely available commercial and free software packages, the techniques of 3D modelling, texturing, sculpting, image editing and animations were combined to create a workflow in the creation of an interactive educational and training tool. This was focussed on cerebral ventricular system anatomy, and the flow of cerebrospinal fluid. Results: We have successfully created a robust methodology by using key software packages in the creation of an interactive education and training tool. This has resulted in an application being developed which details the anatomy of the ventricular system, and flow of cerebrospinal fluid using an anatomically accurate 3D model. -

Diencephalic–Mesencephalic Junction Dysplasia: a Novel Recessive Brain Malformation
doi:10.1093/brain/aws162 Brain 2012: 135; 2416–2427 | 2416 BRAIN A JOURNAL OF NEUROLOGY Diencephalic–mesencephalic junction dysplasia: a novel recessive brain malformation Maha S. Zaki,1 Sahar N. Saleem,2 William B. Dobyns,3 A. James Barkovich,4 Hauke Bartsch,5 Anders M. Dale,5 Manzar Ashtari,6,7 Naiara Akizu,8 Joseph G. Gleeson8 and Ana Maria Grijalvo-Perez8 1 Department of Clinical Genetics, Division of Human Genetics and Genome Research, National Research Centre, Cairo 12311, Egypt 2 Department of Radiology, Cairo University, Cairo, Egypt 3 Department of Paediatrics, Seattle Children’s Research Institute, Seattle, WA 98195-6320, USA 4 Department of Radiology and Biomedical Imaging, University of California, San Francisco, 94143, USA 5 Multimodal Imaging Laboratory (MMIL), Departments of Radiology and Neurosciences, University of California, San Diego, 92093 USA 6 Diffusion Tensor Image Analyses and Brain Morphometry Centre, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA 7 Department of Radiology, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA 8 Neurogenetics Laboratory, Howard Hughes Medical Institute, Department of Neurosciences and Paediatrics, Rady Children’s Hospital, University of California, San Diego, 92093 USA Correspondence to: Dr Maha S. Zaki, Department of Clinical Genetics, Division of Human Genetics and Genome Research, National Research Centre, El-Tahrir Street, Dokki, Cairo 12311, Egypt E-mail: [email protected] or [email protected] We describe six cases from three unrelated consanguineous Egyptian families with a novel characteristic brain malformation at the level of the diencephalic–mesencephalic junction. Brain magnetic resonance imaging demonstrated a dysplasia of the diencephalic–mesencephalic junction with a characteristic ‘butterfly’-like contour of the midbrain on axial sections. -

Neuroanatomy Dr
Neuroanatomy Dr. Maha ELBeltagy Assistant Professor of Anatomy Faculty of Medicine The University of Jordan 2018 Prof Yousry 10/15/17 A F B K G C H D I M E N J L Ventricular System, The Cerebrospinal Fluid, and the Blood Brain Barrier The lateral ventricle Interventricular foramen It is Y-shaped cavity in the cerebral hemisphere with the following parts: trigone 1) A central part (body): Extends from the interventricular foramen to the splenium of corpus callosum. 2) 3 horns: - Anterior horn: Lies in the frontal lobe in front of the interventricular foramen. - Posterior horn : Lies in the occipital lobe. - Inferior horn : Lies in the temporal lobe. rd It is connected to the 3 ventricle by body interventricular foramen (of Monro). Anterior Trigone (atrium): the part of the body at the horn junction of inferior and posterior horns Contains the glomus (choroid plexus tuft) calcified in adult (x-ray&CT). Interventricular foramen Relations of Body of the lateral ventricle Roof : body of the Corpus callosum Floor: body of Caudate Nucleus and body of the thalamus. Stria terminalis between thalamus and caudate. (connects between amygdala and venteral nucleus of the hypothalmus) Medial wall: Septum Pellucidum Body of the fornix (choroid fissure between fornix and thalamus (choroid plexus) Relations of lateral ventricle body Anterior horn Choroid fissure Relations of Anterior horn of the lateral ventricle Roof : genu of the Corpus callosum Floor: Head of Caudate Nucleus Medial wall: Rostrum of corpus callosum Septum Pellucidum Anterior column of the fornix Relations of Posterior horn of the lateral ventricle •Roof and lateral wall Tapetum of the corpus callosum Optic radiation lying against the tapetum in the lateral wall. -

Ultrasound Findings in Neonatal Meningitis—A Pictorial Review
Review Article Neonatal cranial sonography: ultrasound findings in neonatal meningitis—a pictorial review Nishant Gupta1, Hemal Grover2, Itisha Bansal3, Kusum Hooda4, Joshua M. Sapire5, Rama Anand6, Yogesh Kumar7 1Department of Radiology, Saint Vincent’s Medical Center, Bridgeport, CT, USA; 2Department of Neuroradiology, NYU Lagone Medical Center, New York, NY, USA; 3Department of Anesthesiology, New York Methodist Hospital, Brooklyn, New York, USA; 4Department of Radiology, Yale New Haven Health at Bridgeport Hospital, Bridgeport, CT, USA; 5Department of Neuroradiology, Saint Vincent’s Medical Center, Bridgeport, CT, USA; 6Department of Pediatric Radiology, Kalawati Saran Children Hospital, Shaheed Bhagat Singh Marg, New Delhi, India; 7Department of Neuroradiology, Yale New Haven Health at Bridgeport Hospital, Bridgeport, CT, USA Correspondence to: Nishant Gupta, MD, PDCC. Department of Radiology and Imaging, Saint Vincent’s Medical Center, 2800 Main Street, Bridgeport, CT 06606, USA. Email: [email protected]. Abstract: Neonatal bacterial meningitis is a common manifestation of late onset neonatal sepsis. Cranial sonography (CRS) has a crucial role in assessment of infants with clinical suspicion of bacterial meningitis as well as follows up of its complications. CRS is performed with high frequency transducer through anterior fontanelle in both coronal and sagittal planes. Various sonographic findings range from echogenic and widened sulci, ventriculomegaly, ventriculitis, hydrocephalus, extra-axial fluid collections, cerebritis -

Ventriculomegaly
Great Ormond Street Hospital for Children NHS Foundation Trust: Information for Families Ventriculomegaly This information sheet from Great Ormond Street Hospital (GOSH) explains the causes, symptoms and treatment of ventriculomegaly and hydrocephalus and where to get help. Ventricles are cavities within the brain filled Without signs of increased pressure in the with cerebro-spinal fluid (CSF) acting as a brain (hydrocephalus), ventriculomegaly most ‘cushion’. CSF also supplies nutrients to the likely will not cause any problems. However, brain. The brain has four ventricles: two it can be linked with hydrocephalus and other lateral ventricles, the third ventricle and the problems. Ventriculomegaly can be diagnosed fourth ventricle. during pregnancy and occurs in around two CSF is created within the brain and flows from per cent of all pregnancies. the lateral ventricles into the third ventricle. It then flows through a narrow tube (the What causes cerebral aqueduct) into the fourth ventricle which lies towards the base of the brain. From ventriculomegaly? the fourth ventricle, it flows around the spinal In many cases, we do not know what causes cord and over the surface of the brain before ventriculomegaly (in the absence of any raised being re-absorbed. CSF pressure) but it can occur if there has been Ventriculomegaly is the medical term used to brain damage for any reason leading to loss describe enlargement of the ventricles of the of brain tissue. Often however it is a “chance” brain. Hydrocephalus is the term used when finding and when the ventricles are only a enlargement of the ventricles has been caused little enlarged of little significance. -

CT Based Study of Frontal Horn Ratio and Ventricular Index in South Indian Population
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 16, Issue 7 Ver. VI (July. 2017), PP 55-59 www.iosrjournals.org CT Based Study of Frontal Horn Ratio And Ventricular Index in South Indian Population *Dr.Arun Kumar S. MD1, Dr.S.MeenaKumari DMRD.,DNB.2, 3 4 Dr.A.Pavithra DNB. ,Dr.R.Saraswathy DMRD. 1(Associate Professor, Department of Radiology, Karpagam Faculty of Medical Sciences and Research, India) 2(Consultant Radiologist, Department of Radiology, Karpagam Faculty of Medical Sciences and Research, India) 3(Consultant Radiologist, Department of Radiology, Karpagam Faculty of Medical Sciences and Research, India) 4(Consultant Radiologist, Department of Radiology, Karpagam Faculty of Medical Sciences and Research, India) Corresponding author: *Dr. S.MeenaKumari DMRD.,DNB., Abstract: Introduction: Assessment of ventricular morphology and dimensions plays a crucial role in a wide range of clinical conditions associated with ventricular enlargement such as CNS infections, meningitis, and brain tumors . Of all the ventricular dimensions, linear ratios of lateral ventricles are the simplest to enumerate and also to reproduce. Objective: The aim of our study is to establish standard reference values for Frontal Horn Ratio (FHR) and Bicaudate - Frontal Index or Ventricular index using Computed Tomogram (CT) for normal South Indian population. Materials And Methods: One hundred subjects, with normal CT brain, were analyzed for this study retrospectively. Plain CT brain of all the patients was performed in Siemens Somatom Scope Multislice CT scanner. Results: In our study there was no statistically significant difference in mean FHR and ventricular index between genders. -

Estimation of Ventricles Size of Human Brain by Magnetic Resonance
Original Research Article Original Research Article Journal of Gandaki Medical College Nepal Estimation of ventricles size of human brain by Magnetic Resonance Imaging in Nepalese Population: A retrospective study Sushma Singh1* iD , Bhoj Raj Sharma2, Urusha Prajapati3, Pujan Sharma4, Manoj Bhatta3, Nawaraj Poudel2 1Lecturer/B.Sc MIT Programe Coordinator, Department of Radiology Gandaki Medical College 2Lecturer, Department of Radiology Gandaki Medical College 3Radiological Technologist 4Associate Professor, Department of Radiology Gandaki Medical College ABSTRACT Background and Objective: data Magnetic resonance imaging (MRI) provides image acquisition of three-dimensional and measurement in any chosen imaging plane. Objective of this study is to assess the size of ventricles of the dimensions among different age and gender. Materials and methods: This is a cross-sectional retrospective study done brain of normal Nepalese people and establish the range of size of the ventricular system and compute the ventricular at Gandaki Medical College, Pokhara. A total of 106 MRI scan data of healthy individuals were collected over a period of seven months between March to September 2019. Patients ranged between eight and eighty years of age with 58 males and 48 females. Measurements of the mean of bifrontal diameter (BFD), bihemispheric diameter (BHD), third ventricle Result: transverse dimension (TVTD), fourth ventricle antero-posterior dimension (FVAP), fourth ventricle width (FVW), and frontal horn ratio (FHR) were done. The mean of BFD, BHD, TVTD, FVAP, FVW, and FHR were found to be 3.05 ± 0.10 cm, 10.11 ± 0.40 cm, 0.43 ± 0.11 cm, 0.90 ± 0.11 cm, 1.22 ± 0.12 cm, and 0.30 ± 0.01 cm, respectively. -

Morphometric Analysis of Ventricular System of Human Brain - a Study by Dissection Method
Jemds.com Original Research Article Morphometric Analysis of Ventricular System of Human Brain - A Study by Dissection Method Prabahita Baruah1, Purujit Choudhury2, Pradipta Ray Choudhury3 1Department of Anatomy, Silchar Medical College and Hospital, Silchar, Assam, India. 2Department of Surgery, Gauhati Medical College and Hospital, Guwahati, Assam, India. 3Department of Anatomy, Silchar Medical College and Hospital, Silchar, Assam, India. ABSTRACT BACKGROUND It is often a challenge to determine if the brain ventricles are within normal limits Corresponding Author: or swollen with the age of the patient. With a standardized and comparable system, Dr. Purujit Choudhury, it is therefore necessary to define normal ventricular size ranges. Cadaveric Associate Professor, dissection is always considered the gold standard of anatomical education. Present Department of Surgery, Gauhati Medical College and Hospital, work is undertaken to study morphometric analysis of lateral, third & fourth Guwahati, Assam, India. ventricles by dissection method. Morphometric assessment of the ventricular E-mail: [email protected] system is helpful in the diagnosis as well as classification of hydrocephalus and in the evaluation and monitoring of ventricular system enlargement during DOI: 10.14260/jemds/2020/121 ventricular shunt therapy. Financial or Other Competing Interests: METHODS None. Different parameters of all parts of lateral ventricle, third and fourth ventricle were How to Cite This Article: measured with digital vernier caliper in cadaveric brain specimens. The brain Baruah P, Choudhury P, Choudhury PR. specimens were obtained from dead bodies subjected to post-mortem Morphometric analysis of ventricular examinations in the Department of Forensic Medicine and from the dead bodies system of human brain- a study by voluntarily donated to the Department of Anatomy, Silchar Medical College, Silchar. -

Longitudinal Volumetric Assessment of Ventricular Enlargement in Pet Dogs Trained for Functional Magnetic Resonance Imaging (Fmri) Studies
veterinary sciences Article Longitudinal Volumetric Assessment of Ventricular Enlargement in Pet Dogs Trained for Functional Magnetic Resonance Imaging (fMRI) Studies 1, 2, , 2,3 2 4 Eva Gunde y,Kálmán Czeibert * y , Anna Gábor ,Dóra Szabó , Anna Kis , Attila Arany-Tóth 1, Attila Andics 2,3,Márta Gácsi 2,5 and Enik˝oKubinyi 2 1 Department and Clinic of Surgery and Ophthalmology, University of Veterinary Medicine, 1078 Budapest, Hungary; [email protected] (E.G.); [email protected] (A.A.-T.) 2 Department of Ethology, Institute of Biology, ELTE Eötvös Loránd University, 1117 Budapest, Hungary; [email protected] (A.G.); [email protected] (D.S.); [email protected] (A.A.); [email protected] (M.G.); [email protected] (E.K.) 3 MTA-ELTE (Hungarian Academy of Sciences–Eötvös Loránd University) ‘Lendulet¯ Neuroethology of Communication Research Group, 1117 Budapest, Hungary 4 Psychobiology Research Group, Institute of Cognitive Neuroscience and Psychology, Research Centre for Natural Sciences, 1117 Budapest, Hungary; [email protected] 5 MTA-ELTE Comparative Ethology Research Group, 1117 Budapest, Hungary * Correspondence: [email protected] These authors contributed equally to the work reported in this paper. y Received: 4 August 2020; Accepted: 2 September 2020; Published: 4 September 2020 Abstract: Background: Recent studies suggest that clinically sound ventriculomegaly in dogs could be a preliminary form of the clinically significant hydrocephalus. We evaluated changes of ventricular volumes in awake functional magnetic resonance imaging (fMRI) trained dogs with indirectly assessed cognitive abilities over time (thus avoiding the use of anaesthetics, which can alter the pressure). Our research question was whether ventricular enlargement developing over time would have any detrimental effect on staying still while being scanned; which can be extrapolated to the ability to pay attention and to exert inhibition. -

Brain Anatomy
BRAIN ANATOMY Adapted from Human Anatomy & Physiology by Marieb and Hoehn (9th ed.) The anatomy of the brain is often discussed in terms of either the embryonic scheme or the medical scheme. The embryonic scheme focuses on developmental pathways and names regions based on embryonic origins. The medical scheme focuses on the layout of the adult brain and names regions based on location and functionality. For this laboratory, we will consider the brain in terms of the medical scheme (Figure 1): Figure 1: General anatomy of the human brain Marieb & Hoehn (Human Anatomy and Physiology, 9th ed.) – Figure 12.2 CEREBRUM: Divided into two hemispheres, the cerebrum is the largest region of the human brain – the two hemispheres together account for ~ 85% of total brain mass. The cerebrum forms the superior part of the brain, covering and obscuring the diencephalon and brain stem similar to the way a mushroom cap covers the top of its stalk. Elevated ridges of tissue, called gyri (singular: gyrus), separated by shallow groves called sulci (singular: sulcus) mark nearly the entire surface of the cerebral hemispheres. Deeper groves, called fissures, separate large regions of the brain. Much of the cerebrum is involved in the processing of somatic sensory and motor information as well as all conscious thoughts and intellectual functions. The outer cortex of the cerebrum is composed of gray matter – billions of neuron cell bodies and unmyelinated axons arranged in six discrete layers. Although only 2 – 4 mm thick, this region accounts for ~ 40% of total brain mass. The inner region is composed of white matter – tracts of myelinated axons. -
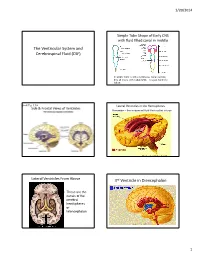
The Ventricular System and Cerebrospinal Fluid (CSF) 3Rd Ventricle in Diencephalon
1/20/2014 Simple Tube Shape of Early CNS with fluid filled canal in middle The Ventricular System and Cerebrospinal Fluid (CSF) In adults there is still a continuous canal running thru all levels of the adult CNS – it is just harder to follow. Book Fig. 1.19 Lateral Ventricles in the Hemispheres Side & Frontal Views of Ventricles Remember – these represent fluid filled cavities in brain “Wishbone” shape means there is ventricle within each of the lobes. Lateral Ventricles From Above 3rd Ventricle in Diencephalon • These are the canals of the cerebral hemispheres or telencephalon 1 1/20/2014 Interventricular foramen links lateral ventricles to 3rd You can see the 2 dark lateral ventricles as well as the vertical midline 3 rd ventricle Sagittal Section Thru 3 rd Vent. (#2 in figure) • Connects to skinny cerebral aqueduct, the canal of the midbrain (just under the #10) Choroid plexus located in ventricles continuously 4th Ventricle in the Hindbrain produces CSF, replacing the ~125-150 ml several times/day Normal pressure 3 holes in the roof of CSF: of the 4 th venticle 100-150 mmH2O allow most CSF to lying down leave ventricles 200-300 mmH2O and enter the sitting up subarachnoid space around brain 2 1/20/2014 Circulation of CSF in • Why is CSF pressure measured in mm H2O? • Larger pressure differences (like BP) are ventricles measured by the movement of a column of always heavier liquid (Mercury or Hg) moves • Smaller pressure differences are measured by the movement of a column of lighter liquid (H2O) posteriorly, – CSF moves that column ~150 mm but would toward only move Hg about 2 mm.